当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dissolution kinetics of citrate coated CoFe2O4 nanoparticles in soil solution
Environmental Science: Nano ( IF 7.3 ) Pub Date : 2022-07-04 , DOI: 10.1039/d2en00330a Yazmin Stefani Perea-Vélez 1 , Ma. del Carmen A. González-Chávez 1 , Rogelio Carrillo-González 2 , Jaime López-Luna 3
Environmental Science: Nano ( IF 7.3 ) Pub Date : 2022-07-04 , DOI: 10.1039/d2en00330a Yazmin Stefani Perea-Vélez 1 , Ma. del Carmen A. González-Chávez 1 , Rogelio Carrillo-González 2 , Jaime López-Luna 3
Affiliation
|
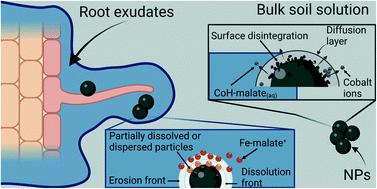
Introducing nanomaterials in agriculture may help to transform the way we farm into a sustainable model by improving the efficiency of fertilizers and reducing the inputs. However, to make sure that these nanomaterials are safe for the environment and their users, the characterization of these nanomaterials and their interactions with the media are needed. Magnetic nanomaterials (such as those based on Fe, Fe oxides, Co-ferrite, etc.) have been investigated for agricultural applications due to their ability to increase the crop yield, photosynthetic rate, and plant biomass. In this context, this research addressed the dissolution rate of citrate-coated CoFe2O4 nanoparticles (NPs) at different pH values (5, 7, and 8) and short periods (to 0.25 until 168 h) in the soil solution of alkaline soil and artificial root exudates. Several equations were tested to fit the rate of dissolution, but the pseudo-second-order model was the best one to fit the release of Co (R2 between 0.80 to 0.99) under all pH soil solution conditions. The k value at pH 5, 7, and 8 was 21.56, 10.52, and −31.33 L mmol−1 h, respectively. Iron was not detected in soil solution experiments; in contrast, artificial root exudates released Fe from NPs. The best models to fit the Fe release from the NPs were the Higuchi and Korsmeyer–Peppas model (R2 >0.92 and R2 adjusted = 0.91). The k values were 4.33 × 10−5 mM h1/2 and 9.88 × 10−3 h−n, respectively. The main species formed from the elements released from the NPs by the ARE action were the complex of Fe and Co with malate (92% of Fe as Fe-malate+, 71% of Co as CoH-malate(aq), and 14% of Co as CoH-malate+). The Fe contained in those complexes may be an Fe exchangeable source for plants. So, due to the poor water solubility of citrate-coated CoFe2O4 NPs and their dissolution by the action of artificial root exudates, these may be considered an option for slow-bio-release Fe fertilizer.
中文翻译:

柠檬酸盐包覆的 CoFe2O4 纳米颗粒在土壤溶液中的溶解动力学
在农业中引入纳米材料可能有助于通过提高肥料效率和减少投入,将我们的耕作方式转变为可持续模式。然而,为了确保这些纳米材料对环境及其用户安全,需要对这些纳米材料及其与介质的相互作用进行表征。磁性纳米材料(例如基于 Fe、Fe 氧化物、Co-铁氧体等的材料)已被研究用于农业应用,因为它们能够提高作物产量、光合速率和植物生物量。在此背景下,本研究探讨了柠檬酸盐涂层 CoFe 2 O 4的溶解速率在碱性土壤和人工根系分泌物的土壤溶液中,不同 pH 值(5、7 和 8)和短期(至 0.25 至 168 小时)的纳米颗粒 (NPs)。测试了几个方程来拟合溶解速率,但伪二级模型是拟合所有 pH 土壤溶液条件下Co ( R 2介于 0.80 到 0.99 之间) 释放的最佳模型。pH 5、7 和 8 时的k值分别为 21.56、10.52 和 -31.33 L mmol -1 h。土壤溶液实验未检出铁;相反,人工根系分泌物从 NPs 中释放出 Fe。适合从 NP 释放 Fe 的最佳模型是 Higuchi 和 Korsmeyer-Peppas 模型(R 2 >0.92 和R 2调整 = 0.91)。k值分别为4.33 × 10 -5 mM h 1/2和9.88 × 10 -3 h - n。由 ARE 作用从 NPs 释放的元素形成的主要物质是 Fe 和 Co 与苹果酸的络合物(92% 的 Fe 为 Fe-苹果酸+,71% 的 Co 为 CoH-苹果酸(aq)和 14%的 CoH-苹果酸+ )。这些配合物中所含的铁可能是植物的铁交换来源。因此,由于柠檬酸盐涂层的 CoFe 2 O 4水溶性差NPs 及其通过人工根系分泌物的作用溶解,这些可能被认为是生物缓释铁肥的一种选择。
更新日期:2022-07-04
中文翻译:

柠檬酸盐包覆的 CoFe2O4 纳米颗粒在土壤溶液中的溶解动力学
在农业中引入纳米材料可能有助于通过提高肥料效率和减少投入,将我们的耕作方式转变为可持续模式。然而,为了确保这些纳米材料对环境及其用户安全,需要对这些纳米材料及其与介质的相互作用进行表征。磁性纳米材料(例如基于 Fe、Fe 氧化物、Co-铁氧体等的材料)已被研究用于农业应用,因为它们能够提高作物产量、光合速率和植物生物量。在此背景下,本研究探讨了柠檬酸盐涂层 CoFe 2 O 4的溶解速率在碱性土壤和人工根系分泌物的土壤溶液中,不同 pH 值(5、7 和 8)和短期(至 0.25 至 168 小时)的纳米颗粒 (NPs)。测试了几个方程来拟合溶解速率,但伪二级模型是拟合所有 pH 土壤溶液条件下Co ( R 2介于 0.80 到 0.99 之间) 释放的最佳模型。pH 5、7 和 8 时的k值分别为 21.56、10.52 和 -31.33 L mmol -1 h。土壤溶液实验未检出铁;相反,人工根系分泌物从 NPs 中释放出 Fe。适合从 NP 释放 Fe 的最佳模型是 Higuchi 和 Korsmeyer-Peppas 模型(R 2 >0.92 和R 2调整 = 0.91)。k值分别为4.33 × 10 -5 mM h 1/2和9.88 × 10 -3 h - n。由 ARE 作用从 NPs 释放的元素形成的主要物质是 Fe 和 Co 与苹果酸的络合物(92% 的 Fe 为 Fe-苹果酸+,71% 的 Co 为 CoH-苹果酸(aq)和 14%的 CoH-苹果酸+ )。这些配合物中所含的铁可能是植物的铁交换来源。因此,由于柠檬酸盐涂层的 CoFe 2 O 4水溶性差NPs 及其通过人工根系分泌物的作用溶解,这些可能被认为是生物缓释铁肥的一种选择。



























 京公网安备 11010802027423号
京公网安备 11010802027423号