Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvatochromic Cellular Stress Sensors Reveal the Compactness Heterogeneity and Dynamics of Aggregated Proteome
ACS Sensors ( IF 8.2 ) Pub Date : 2022-07-01 , DOI: 10.1021/acssensors.2c00566 Qiuxuan Xia 1, 2 , Wang Wan 1 , Wenhan Jin 1 , Yanan Huang 1 , Rui Sun 1, 2 , Mengdie Wang 1, 2 , Biao Jing 1, 3 , Congcong Peng 1, 3 , Xuepeng Dong 3 , Rixin Zhang 3 , Zhenming Gao 3 , Yu Liu 1
ACS Sensors ( IF 8.2 ) Pub Date : 2022-07-01 , DOI: 10.1021/acssensors.2c00566 Qiuxuan Xia 1, 2 , Wang Wan 1 , Wenhan Jin 1 , Yanan Huang 1 , Rui Sun 1, 2 , Mengdie Wang 1, 2 , Biao Jing 1, 3 , Congcong Peng 1, 3 , Xuepeng Dong 3 , Rixin Zhang 3 , Zhenming Gao 3 , Yu Liu 1
Affiliation
|
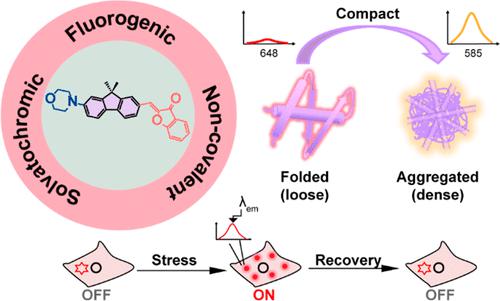
Deterioration of protein homeostasis (proteostasis) often induces aberrant proteome aggregation. Visualization and dissection of the stressed proteome are of particular interest given their association with numerous degenerative diseases. Recent progress in chemical cellular stress sensors allows for direct visualization of aggregated proteome. Beyond its localization and morphology, the physicochemical nature and the dynamics of the aggregated proteome have been challenging to explore. Herein, we developed a series of solvatochromic fluorene-based D-π-A probes that can selectively and noncovalently bind to a misfolded and aggregated proteome and report on their compactness heterogeneity upon cellular stresses. We achieved this goal by variation of the heterocyclic acceptors to modulate their solvatochromism and binding affinity to amorphous aggregated proteins. The optimized sensor P6 was capable of sensing the polarity differences among different aggregated proteins via its fluorescence emission wavelength. In live cells, P6 revealed the cellular compactness heterogeneity in the aggregated proteome upon cellular stresses. Given the combinative solvatochromic and noncovalent properties, our probe can reversibly monitor the dynamic changes in the aggregated proteome compactness upon stress and after stress recovery, suggesting its potential applications in search of therapeutics to counteract disease-causing proteome stresses.
中文翻译:

Solvatochromic 细胞应力传感器揭示了聚集蛋白质组的紧凑性异质性和动力学
蛋白质稳态(proteostasis)的恶化通常会导致异常的蛋白质组聚集。应激蛋白质组的可视化和解剖特别令人感兴趣,因为它们与许多退行性疾病有关。化学细胞应力传感器的最新进展允许直接可视化聚合蛋白质组。除了其定位和形态之外,聚合蛋白质组的物理化学性质和动力学一直难以探索。在此,我们开发了一系列溶剂化变色芴基 D- π-一种探针,可以选择性地和非共价地结合错误折叠和聚集的蛋白质组,并报告它们在细胞压力下的紧密性异质性。我们通过改变杂环受体来调节它们的溶剂化显色性和与无定形聚集蛋白的结合亲和力来实现这一目标。优化后的传感器 P6 能够通过其荧光发射波长感知不同聚集蛋白之间的极性差异。在活细胞中,P6 揭示了在细胞应激下聚合蛋白质组中的细胞紧密度异质性。鉴于组合的溶剂致变色和非共价性质,我们的探针可以可逆地监测聚合蛋白质组紧密度在压力和压力恢复后的动态变化,
更新日期:2022-07-01
中文翻译:

Solvatochromic 细胞应力传感器揭示了聚集蛋白质组的紧凑性异质性和动力学
蛋白质稳态(proteostasis)的恶化通常会导致异常的蛋白质组聚集。应激蛋白质组的可视化和解剖特别令人感兴趣,因为它们与许多退行性疾病有关。化学细胞应力传感器的最新进展允许直接可视化聚合蛋白质组。除了其定位和形态之外,聚合蛋白质组的物理化学性质和动力学一直难以探索。在此,我们开发了一系列溶剂化变色芴基 D- π-一种探针,可以选择性地和非共价地结合错误折叠和聚集的蛋白质组,并报告它们在细胞压力下的紧密性异质性。我们通过改变杂环受体来调节它们的溶剂化显色性和与无定形聚集蛋白的结合亲和力来实现这一目标。优化后的传感器 P6 能够通过其荧光发射波长感知不同聚集蛋白之间的极性差异。在活细胞中,P6 揭示了在细胞应激下聚合蛋白质组中的细胞紧密度异质性。鉴于组合的溶剂致变色和非共价性质,我们的探针可以可逆地监测聚合蛋白质组紧密度在压力和压力恢复后的动态变化,











































 京公网安备 11010802027423号
京公网安备 11010802027423号