Journal of Advanced Research ( IF 11.4 ) Pub Date : 2022-06-29 , DOI: 10.1016/j.jare.2022.06.011 Bingling Zhong 1 , Lin Zhao 1 , Jie Yu 1 , Ying Hou 1 , Nana Ai 2 , Jin-Jian Lu 3 , Wei Ge 2 , Xiuping Chen 3
|
Introduction
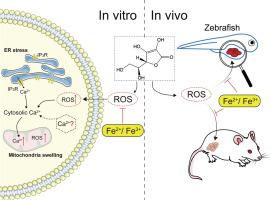
The anti-cancer effect of high concentrations of ascorbic acid (AA) has been well established while its underlying mechanisms remain unclear. The association between iron and AA has attracted great attention but was still controversial due to the complicated roles of iron in tumors.
Objectives
Our study aims to explore the anti-cancer mechanisms of AA and the interaction between AA and iron in cancer.
Methods
The MTT and ATP assays were used to evaluate the cytotoxicity of AA. Reactive oxygen species (ROS) generation, calcium (Ca2+), and lipid peroxidation were monitored with flow cytometry. Mitochondrial dysfunction was assessed by mitochondrial membrane potential (MMP) detection with JC-1 or tetramethylrhodamine methyl ester (TMRM) staining. Mitochondrial swelling was monitored with MitoTracker Green probe. FeSO4 (Fe2+), FeCl3 (Fe3+), Ferric ammonium citrate (Fe3+), hemin chloride (Fe3+) were used as an iron donor to investigate the effects of iron on AA’s anti-tumor activity. The in vivo effects of AA and iron were analyzed in xenograft zebrafish and allograft mouse models.
Results
High concentrations of AA exhibited cytotoxicity in a panel of cancer cells. AA triggered ROS-dependent non-apoptotic cell death. AA-induced cell death was essentially mediated by the accumulated intracellular Ca2+, which was partly originated from endoplasmic reticulum (ER). Surprisingly, exogenous iron could significantly reverse AA-induced ROS generation, Ca2+ overloaded, and cell death. Especially, the iron supplements significantly impaired the in vivo anti-tumor activity of AA.
Conclusions
Our study elucidated the protective roles of iron in ROS/Ca2+ mediated necrosis triggered by AA both in vitro and in vivo, which might shed novel insight into the anti-cancer mechanisms and provide clinical application strategies for AA in cancer treatment.
中文翻译:

外源性铁损害抗坏血酸的体外和体内抗癌作用
介绍
高浓度抗坏血酸 (AA) 的抗癌作用已得到充分证实,但其潜在机制仍不清楚。铁和 AA 之间的关联引起了极大的关注,但由于铁在肿瘤中的复杂作用,仍然存在争议。
目标
我们的研究旨在探索AA的抗癌机制以及AA与铁在癌症中的相互作用。
方法
MTT 和 ATP 测定用于评估 AA 的细胞毒性。使用流式细胞仪监测活性氧 (ROS) 生成、钙 (Ca 2+ ) 和脂质过氧化。线粒体功能障碍通过使用 JC-1 或四甲基罗丹明甲酯 (TMRM) 染色的线粒体膜电位 (MMP) 检测来评估。用 MitoTracker Green 探针监测线粒体肿胀。以FeSO 4 (Fe 2+ )、FeCl 3 (Fe 3+ )、柠檬酸铁铵(Fe 3+ )、氯化血红素(Fe 3+ )为铁供体,研究铁对AA抗肿瘤活性的影响. 在体内在异种移植斑马鱼和同种异体移植小鼠模型中分析了 AA 和铁的作用。
结果
高浓度的 AA 在一组癌细胞中表现出细胞毒性。AA 引发 ROS 依赖性非凋亡性细胞死亡。AA 诱导的细胞死亡主要由累积的细胞内 Ca 2+介导,其部分源自内质网 (ER)。令人惊讶的是,外源性铁可以显着逆转 AA 诱导的 ROS 生成、Ca 2+超载和细胞死亡。特别是,铁补充剂显着损害了AA 的体内抗肿瘤活性。
结论
我们的研究阐明了铁在体外和体内由 AA 引发的ROS/Ca 2+介导的坏死中的保护作用,这可能为抗癌机制提供新的见解,并为 AA 在癌症治疗中的临床应用提供策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号