当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering of reaction specificity, enantioselectivity, and catalytic activity of nitrilase for highly efficient synthesis of pregabalin precursor
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2022-06-24 , DOI: 10.1002/bit.28165 Xia-Feng Lu 1, 2 , Hong-Juan Diao 1, 2 , Zhe-Ming Wu 1, 2 , Zi-Long Zhang 1 , Ren-Chao Zheng 1, 2 , Yu-Guo Zheng 1, 2
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2022-06-24 , DOI: 10.1002/bit.28165 Xia-Feng Lu 1, 2 , Hong-Juan Diao 1, 2 , Zhe-Ming Wu 1, 2 , Zi-Long Zhang 1 , Ren-Chao Zheng 1, 2 , Yu-Guo Zheng 1, 2
Affiliation
|
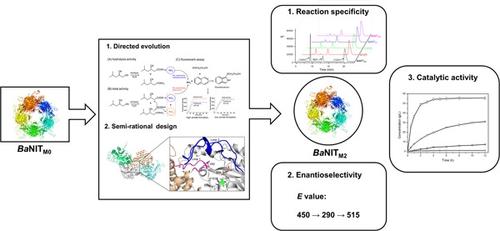
Simultaneous evolution of multiple enzyme properties remains challenging in protein engineering. A chimeric nitrilase (BaNITM0) with high activity towards isobutylsuccinonitrile (IBSN) was previously constructed for biosynthesis of pregabalin precursor (S)-3-cyano-5-methylhexanoic acid ((S)-CMHA). However, BaNITM0 also catalyzed the hydration of IBSN to produce by-product (S)-3-cyano-5-methylhexanoic amide. To obtain industrial nitrilase with vintage performance, we carried out engineering of BaNITM0 for simultaneous evolution of reaction specificity, enantioselectivity, and catalytic activity. The best variant V82L/M127I/C237S (BaNITM2) displayed higher enantioselectivity (E = 515), increased enzyme activity (5.4-fold) and reduced amide formation (from 15.8% to 1.9%) compared with BaNITM0. Structure analysis and molecular dynamics simulations indicated that mutation M127I and C237S restricted the movement of E66 in the catalytic triad, resulting in decreased amide formation. Mutation V82L was incorporated to induce the reconstruction of the substrate binding region in the enzyme catalytic pocket, engendering the improvement of stereoselectivity. Enantio- and regio-selective hydrolysis of 150 g/L IBSN using 1.5 g/L Escherichia coli cells harboring BaNITM2 as biocatalyst afforded (S)-CMHA with >99.0% ee and 45.9% conversion, which highlighted the robustness of BaNITM2 for efficient manufacturing of pregabalin.
中文翻译:

用于高效合成普瑞巴林前体的腈水解酶的反应特异性、对映选择性和催化活性工程
多种酶特性的同时进化在蛋白质工程中仍然具有挑战性。先前构建了对异丁基丁二腈 (IBSN) 具有高活性的嵌合腈水解酶 ( Ba NIT M0 ),用于生物合成普瑞巴林前体 ( S )-3-氰基-5-甲基己酸 (( S )-CMHA)。然而,Ba NIT M0也催化 IBSN 水合生成副产物 ( S )-3-cyano-5-methylhexanoic amide。为了获得具有复古性能的工业腈水解酶,我们对Ba NIT M0进行了工程化用于同时进化反应特异性、对映选择性和催化活性。与Ba NIT M0相比,最佳变体 V82L/M127I/C237S ( Ba NIT M2 ) 表现出更高的对映选择性 ( E = 515)、增加的酶活性 (5.4 倍) 和减少的酰胺形成 (从 15.8% 到 1.9%). 结构分析和分子动力学模拟表明突变M127I和C237S限制了E66在催化三联体中的运动,导致酰胺形成减少。掺入突变V82L以诱导酶催化袋中底物结合区域的重建,从而提高立体选择性。使用含有Ba NIT M2的 1.5 g/L大肠杆菌细胞作为生物催化剂对 150 g/L IBSN 进行对映和区域选择性水解,得到 ( S )-CMHA,具有 >99.0% ee和 45.9% 的转化率,这突出了Ba NIT的稳健性M2用于高效生产普瑞巴林。
更新日期:2022-06-24
中文翻译:

用于高效合成普瑞巴林前体的腈水解酶的反应特异性、对映选择性和催化活性工程
多种酶特性的同时进化在蛋白质工程中仍然具有挑战性。先前构建了对异丁基丁二腈 (IBSN) 具有高活性的嵌合腈水解酶 ( Ba NIT M0 ),用于生物合成普瑞巴林前体 ( S )-3-氰基-5-甲基己酸 (( S )-CMHA)。然而,Ba NIT M0也催化 IBSN 水合生成副产物 ( S )-3-cyano-5-methylhexanoic amide。为了获得具有复古性能的工业腈水解酶,我们对Ba NIT M0进行了工程化用于同时进化反应特异性、对映选择性和催化活性。与Ba NIT M0相比,最佳变体 V82L/M127I/C237S ( Ba NIT M2 ) 表现出更高的对映选择性 ( E = 515)、增加的酶活性 (5.4 倍) 和减少的酰胺形成 (从 15.8% 到 1.9%). 结构分析和分子动力学模拟表明突变M127I和C237S限制了E66在催化三联体中的运动,导致酰胺形成减少。掺入突变V82L以诱导酶催化袋中底物结合区域的重建,从而提高立体选择性。使用含有Ba NIT M2的 1.5 g/L大肠杆菌细胞作为生物催化剂对 150 g/L IBSN 进行对映和区域选择性水解,得到 ( S )-CMHA,具有 >99.0% ee和 45.9% 的转化率,这突出了Ba NIT的稳健性M2用于高效生产普瑞巴林。











































 京公网安备 11010802027423号
京公网安备 11010802027423号