JACC: Heart Failure ( IF 10.3 ) Pub Date : 2022-06-27 , DOI: 10.1016/j.jchf.2022.04.013 Justin M Vader 1 , Michael M Givertz 2 , Randall C Starling 3 , Steven E McNulty 4 , Kevin J Anstrom 4 , Patrice Desvigne-Nickens 5 , Adrian F Hernandez 6 , Eugene Braunwald 2 , Douglas L Mann 1 ,
|
Background
The LIFE (LCZ696 In Hospitalized Advanced Heart FailurE) trial, which evaluated sacubitril/valsartan in patients with advanced heart failure (HF) with reduced ejection fraction and recent New York Heart Association functional class IV symptomatology, did not require tolerance to a renin angiotensin system antagonist before initiating sacubitril/valsartan, thus affording an opportunity to study the tolerability of sacubitril/valsartan in advanced HF with reduced ejection fraction.
Objectives
The goal of this analysis of the LIFE trial is to characterize the tolerability of initiating sacubitril/valsartan in patients with chronic advanced HF with reduced ejection fraction.
Methods
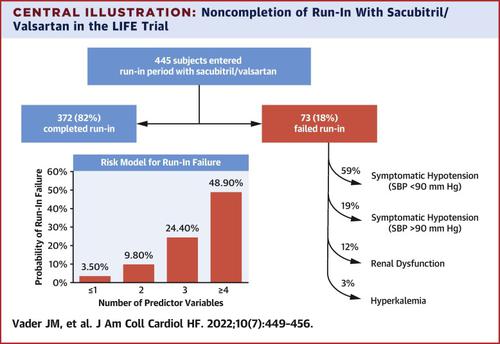
In the LIFE trial, 445 subjects with advanced HF entered an unblinded run-in period of 3-7 days with sacubitril/valsartan 24/26 mg twice a day. The authors compared characteristics of subjects completing and failing run-in, performed multivariable analysis of clinical parameters associated with run-in failure, and developed a predictive model for short-term intolerance to sacubitril/valsartan.
Results
Of 445 subjects entering run-in, 73 (18%) were intolerant of sacubitril/valsartan. Reasons for intolerance included systolic blood pressure <90 mm Hg (59%), symptoms of hypotension/dizziness with systolic blood pressure >90 mm Hg (19%), and renal dysfunction (creatinine >2.0 mg/dL) (12%). Multivariable predictors of intolerance included lower mean arterial pressure, lower serum chloride, presence of an implantable cardioverter-defibrillator and/or cardiac resynchronization device, moderate or greater mitral regurgitation, nonuse of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker at the screening visit, and use of insulin at screening. Subjects with 4 or more predictors had a 48.9% probability of sacubitril/valsartan intolerance.
Conclusions
Intolerance to low doses of sacubitril/valsartan is common in patients with advanced chronic HF with reduced ejection fraction and may be predicted by the presence of certain risk factors. (EntrestoTM [LCZ696] in Advanced Heart Failure [LIFE Study] [HFN-LIFE] NCT02816736)
中文翻译:

晚期心力衰竭患者对沙库巴曲/缬沙坦的耐受性
背景
LIFE (LCZ696 In Hospitalized Advanced Heart FailurE) 试验评估了沙库巴曲/缬沙坦在射血分数降低的晚期心力衰竭 (HF) 患者和最近纽约心脏协会功能分级 IV 症状学中的疗效,不需要对肾素血管紧张素系统耐受在开始沙库巴曲/缬沙坦之前,拮抗剂,从而提供了一个机会来研究沙库巴曲/缬沙坦在射血分数降低的晚期 HF 中的耐受性。
目标
这项 LIFE 试验分析的目的是表征射血分数降低的慢性晚期 HF 患者开始使用沙库巴曲/缬沙坦的耐受性。
方法
在 LIFE 试验中,445 名患有晚期 HF 的受试者进入了为期 3-7 天的非盲磨合期,每天两次服用 sacubitril/valsartan 24/26 mg。作者比较了完成磨合和失败磨合的受试者的特征,对与磨合失败相关的临床参数进行了多变量分析,并开发了沙库巴曲/缬沙坦短期不耐受的预测模型。
结果
在进入磨合期的 445 名受试者中,73 名 (18%) 对沙库巴曲/缬沙坦不耐受。不耐受的原因包括收缩压 <90 毫米汞柱 (59%)、低血压/头晕症状伴收缩压 >90 毫米汞柱 (19%) 和肾功能障碍(肌酐 >2.0 毫克/分升)(12%)。不耐受的多变量预测因素包括较低的平均动脉压、较低的血清氯化物、植入式心律转复除颤器和/或心脏再同步装置的存在、中度或更大程度的二尖瓣反流、筛选访视时未使用血管紧张素转换酶抑制剂或血管紧张素受体阻滞剂、筛选时使用胰岛素。有 4 个或更多预测因子的受试者有 48.9% 的概率为 sacubitril/valsartan 不耐受。
结论
对低剂量沙库巴曲/缬沙坦的不耐受在射血分数降低的晚期慢性 HF 患者中很常见,并且可以通过某些风险因素的存在来预测。(用于晚期心力衰竭的 EntrestoTM [LCZ696] [LIFE 研究] [HFN-LIFE] NCT02816736)











































 京公网安备 11010802027423号
京公网安备 11010802027423号