当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Molecular Chains Arrangement and Surface Energy State in the Low Ice Adhesion on Poly(tetrafluoroethylene)
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2022-06-27 , DOI: 10.1021/acs.jpclett.2c01331 Yangjiangshan Xu 1 , Yizhou Shen 1 , Jie Tao 1 , Jiawei Jiang 1 , Weilan Liu 1, 2 , Haifeng Chen 3 , Senyun Liu 4 , Huaguan Li 5 , Biao Jiang 1 , Xinyu Xie 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2022-06-27 , DOI: 10.1021/acs.jpclett.2c01331 Yangjiangshan Xu 1 , Yizhou Shen 1 , Jie Tao 1 , Jiawei Jiang 1 , Weilan Liu 1, 2 , Haifeng Chen 3 , Senyun Liu 4 , Huaguan Li 5 , Biao Jiang 1 , Xinyu Xie 1
Affiliation
|
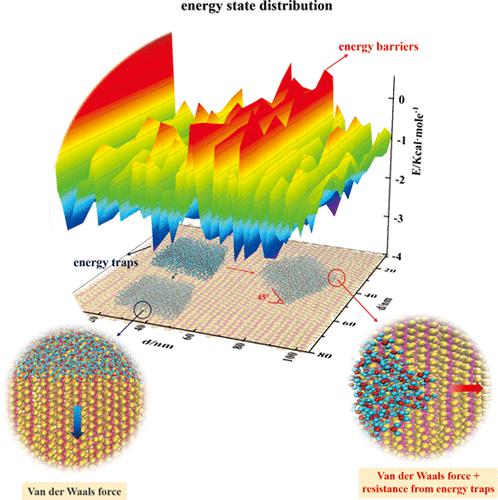
The relation between polymer molecular chains arrangement and ice adhesion was studied at the molecular scale, and the energy states of water molecules on the poly(tetrafluoroethylene) surface were analyzed to explain the energy essence of ice adhesion. The ice adhesion on crystalline poly(tetrafluoroethylene) displayed a clear anisotropy phenomenon. Further research proved that the energy states of water molecules along the vertical direction of the molecular chains fluctuated regularly, and the water molecules in gaps between molecular chains were in the energy troughs, leading to the formation of energy traps. Water molecules needed more energy from outside to escape the energy traps, causing additional resistance to the ice movement and obvious increase of ice adhesion. Therefore, ice adhesion was closely related to the distribution of energy traps in the direction of ice removing, which mainly depended on the possibility of molecular chains perpendicularly arranged in the direction of ice removing.
中文翻译:

分子链排列和表面能状态在聚四氟乙烯低冰粘附中的作用
从分子尺度研究聚合物分子链排列与冰附着的关系,分析聚四氟乙烯表面水分子的能态,解释冰附着的能量本质。结晶聚(四氟乙烯)上的冰粘附表现出明显的各向异性现象。进一步研究证明,水分子沿分子链垂直方向的能态有规律地波动,分子链间隙中的水分子处于能量谷中,导致能量陷阱的形成。水分子需要更多来自外部的能量才能逃离能量陷阱,从而对冰的运动造成额外的阻力,并明显增加冰的附着力。所以,
更新日期:2022-06-27
中文翻译:

分子链排列和表面能状态在聚四氟乙烯低冰粘附中的作用
从分子尺度研究聚合物分子链排列与冰附着的关系,分析聚四氟乙烯表面水分子的能态,解释冰附着的能量本质。结晶聚(四氟乙烯)上的冰粘附表现出明显的各向异性现象。进一步研究证明,水分子沿分子链垂直方向的能态有规律地波动,分子链间隙中的水分子处于能量谷中,导致能量陷阱的形成。水分子需要更多来自外部的能量才能逃离能量陷阱,从而对冰的运动造成额外的阻力,并明显增加冰的附着力。所以,











































 京公网安备 11010802027423号
京公网安备 11010802027423号