Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Indole-substituted flavonol-based cysteine fluorescence sensing and subsequent precisely controlled linear CO liberation
Analyst ( IF 4.2 ) Pub Date : 2022-06-28 , DOI: 10.1039/d2an00631f Ying-Ji Sun 1 , Deng-Jie Zhao 1 , Bo Song 1
Analyst ( IF 4.2 ) Pub Date : 2022-06-28 , DOI: 10.1039/d2an00631f Ying-Ji Sun 1 , Deng-Jie Zhao 1 , Bo Song 1
Affiliation
|
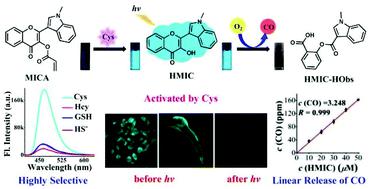
The first water-soluble B-ring-indole-substituted flavonol-based cysteine (Cys) fluorescent probe, MICA (2-(1-methyl-1H-indol-3-yl)-4-oxo-4H-chromen-3-yl-acrylate), was developed, which simultaneously serves as a precursor of photoCORM. In PBS buffer (only 15% DMF), MICA can perform rapid (330 s), highly chemoselective (particularly for homocysteine and glutathione) and sensitive (limit of detection: 92 nM) sensing and visualization of exogenous and endogenous Cys in live HeLa cells and zebrafish over a wide linear concentration range (0–12 μM/2.4 equiv.). The fluorophore HMIC (3-hydroxy-2-(1-methyl-1H-indol-3-yl)-4H-chromen-4-one), actuated and quantitatively generated via the sensing reaction of the precursor MICA with Cys, was designed as a photoCORM. By modulating the light illumination intensity or illumination duration or photoCORM dosage, HMIC can provide precisely controlled quantitative and linear CO gas by visible light illumination in aerobic environments. For live HeLa cells, MICA and all reaction products showed low toxicity (over 85% cell viability versus 10 μM analyst) and efficient cellular uptake. In live HeLa cells and zebrafish, both exogenous and endogenous Cys can be visualized by MICA, and the location and CO liberation process of the generated HMIC can be tracked in real time through its fluorescence. Substitution of the B-ring of 3-hydroxy-flavone (3-FL) by indole results in a 52 nm absorption red-shift vs. 3-FL. Our work is the first water-soluble B-ring-indole-substituted flavonol-based fluorescent probe that efficaciously detects and visualizes exogenous and endogenous Cys both in vitro and in vivo, simultaneously serving as a precursor of photoCORM, actuated by Cys and triggered by visible light, releasing linear CO in aerobic environments. This work not only provides promising applications for the detection and visualization of exogenous and endogenous Cys, and spatiotemporally controllable CO liberation in live systems, but will also facilitate the development of handy molecular tools for clinical diagnosis and CO gas therapy.
中文翻译:

基于吲哚取代黄酮醇的半胱氨酸荧光传感和随后精确控制的线性 CO 释放
第一个水溶性 B 环吲哚取代黄酮醇基半胱氨酸 (Cys) 荧光探针MICA (2-(1-methyl-1 H -indol-3-yl)-4-oxo-4 H -chromen- 3-丙烯酸酯),它同时作为 photoCORM 的前体。在 PBS 缓冲液(仅 15% DMF)中,MICA可以在活 HeLa 细胞中执行快速(330 秒)、高度化学选择性(特别是同型半胱氨酸和谷胱甘肽)和灵敏(检测限:92 nM)传感和可视化外源性和内源性半胱氨酸和斑马鱼在很宽的线性浓度范围内(0–12 μM/2.4 equiv.)。荧光团HMIC (3-hydroxy-2-(1-methyl-1 H -indol-3-yl)-4 H-chromen-4-one),通过前体MICA与 Cys 的传感反应驱动和定量产生,被设计为 photoCORM。通过调节光照强度或光照持续时间或 photoCORM 剂量,HMIC可以在有氧环境中通过可见光照射提供精确控制的定量和线性 CO 气体。对于活 HeLa 细胞,MICA和所有反应产物均显示出低毒性(与10 μM 分析剂相比,细胞活力超过 85%)和有效的细胞摄取。在活 HeLa 细胞和斑马鱼中,外源性和内源性 Cys 都可以通过MICA可视化,以及生成的HMIC的位置和 CO 释放过程可以通过其荧光实时跟踪。用吲哚取代 3-羟基黄酮 ( 3-FL ) 的 B 环导致 52 nm 吸收相对于 3-FL发生红移。我们的工作是第一个水溶性 B 环吲哚取代黄酮醇基荧光探针,可在体外和体内有效检测和可视化外源性和内源性 Cys,同时作为 photoCORM 的前体,由 Cys 驱动并由可见光触发,在有氧环境中释放线性 CO。这项工作不仅为外源性和内源性 Cys 的检测和可视化以及活体系统中时空可控的 CO 释放提供了有前景的应用,而且还将促进用于临床诊断和 CO 气体治疗的便捷分子工具的开发。
更新日期:2022-06-28
中文翻译:

基于吲哚取代黄酮醇的半胱氨酸荧光传感和随后精确控制的线性 CO 释放
第一个水溶性 B 环吲哚取代黄酮醇基半胱氨酸 (Cys) 荧光探针MICA (2-(1-methyl-1 H -indol-3-yl)-4-oxo-4 H -chromen- 3-丙烯酸酯),它同时作为 photoCORM 的前体。在 PBS 缓冲液(仅 15% DMF)中,MICA可以在活 HeLa 细胞中执行快速(330 秒)、高度化学选择性(特别是同型半胱氨酸和谷胱甘肽)和灵敏(检测限:92 nM)传感和可视化外源性和内源性半胱氨酸和斑马鱼在很宽的线性浓度范围内(0–12 μM/2.4 equiv.)。荧光团HMIC (3-hydroxy-2-(1-methyl-1 H -indol-3-yl)-4 H-chromen-4-one),通过前体MICA与 Cys 的传感反应驱动和定量产生,被设计为 photoCORM。通过调节光照强度或光照持续时间或 photoCORM 剂量,HMIC可以在有氧环境中通过可见光照射提供精确控制的定量和线性 CO 气体。对于活 HeLa 细胞,MICA和所有反应产物均显示出低毒性(与10 μM 分析剂相比,细胞活力超过 85%)和有效的细胞摄取。在活 HeLa 细胞和斑马鱼中,外源性和内源性 Cys 都可以通过MICA可视化,以及生成的HMIC的位置和 CO 释放过程可以通过其荧光实时跟踪。用吲哚取代 3-羟基黄酮 ( 3-FL ) 的 B 环导致 52 nm 吸收相对于 3-FL发生红移。我们的工作是第一个水溶性 B 环吲哚取代黄酮醇基荧光探针,可在体外和体内有效检测和可视化外源性和内源性 Cys,同时作为 photoCORM 的前体,由 Cys 驱动并由可见光触发,在有氧环境中释放线性 CO。这项工作不仅为外源性和内源性 Cys 的检测和可视化以及活体系统中时空可控的 CO 释放提供了有前景的应用,而且还将促进用于临床诊断和 CO 气体治疗的便捷分子工具的开发。



























 京公网安备 11010802027423号
京公网安备 11010802027423号