Water Research ( IF 11.4 ) Pub Date : 2022-06-26 , DOI: 10.1016/j.watres.2022.118799 Ling Zhu 1 , Xiaoxian Zhang 1 , Jichen Zhang 1 , Tingran Liu 1 , Yuping Qiu 1
|
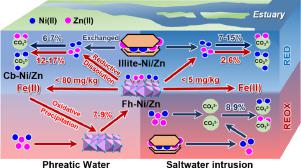
Iron in the form of (oxyhydr)oxides plays a profound role in the (im)mobilization of heavy metals in environmental geochemical processes occurring in the soil–groundwater system. Here, the influence of saltwater intrusion on Fe-(oxyhydr)oxide-mediated (im)mobilization of Ni(II) and Zn(II) in redox-fluctuating shallow aquifers was evaluated by chemical extraction, μ-XRF-XANES analysis, and 16S rRNA high-throughput sequencing. In phreatic water, the ferrihydrite-bound Ni/Zn (Fh-Ni/Zn) in soils contributed to a 12%–17% increase in carbonate-bound Ni/Zn (Cb-Ni/Zn) due to its own reductive dissolution, whereas the illite-adsorbed Ni/Zn (illite-Ni/Zn) only contributed 6%, 7%. The relative abundance of non-salt tolerant anaerobic Herbaspirillum and iron-reducing associated Ralstonia in soils accounted for nearly 50%. During the oxidation stage, the dissolved ferrihydrite reprecipitated to bind free Ni/Zn. However, saltwater invasion strongly weakened the dissolution–precipitation of ferrihydrite by inhibiting the growth of non-salt tolerant anaerobes and iron-reducing bacteria, and highlighted the contribution of illite-Ni/Zn. Under brackish water intrusion, illite-Zn contributed to a 12% increase in Cb-Zn, thereby surpassing the contribution of Fh-Zn (8%). Under seawater invasion, the dissolution–precipitation of ferrihydrite hardly occurred and the anaerobic salt-tolerant Bacillus (> 95%) prevailed. Therefore, the increase of Cb-Ni/Zn (7%–15%) in the reduction stages was contributed by illite-Ni/Zn. However, in the oxidation stages, the carbonate replaced the original role of reprecipitated ferrihydrite to bind the free Ni/Zn in solutions. These newly recognized mechanisms may be the key to predicting the mobility of toxic elements and developing appropriate remediation techniques of permeable reactive barriers under salinity stress.
中文翻译:

盐水侵入削弱了氧化还原波动的土壤 - 地下水系统中铁(氧氢)氧化物介导的镍和锌的(固定)迁移
(氢氧化物)氧化物形式的铁在土壤-地下水系统中发生的环境地球化学过程中的重金属(固定)固定中发挥着重要作用。在这里,通过化学萃取、μ-XRF-XANES 分析和16S rRNA 高通量测序。在潜水中,由于其自身的还原溶解,土壤中水铁矿结合的镍/锌(Fh-Ni/Zn)导致碳酸盐结合的镍/锌(Cb-Ni/Zn)增加了 12%–17%,而伊利石吸附的镍/锌(伊利石-镍/锌)仅贡献了6%、7%。非耐盐厌氧Herbaspirillum和铁还原相关的Ralstonia的相对丰度土壤中占近50%。在氧化阶段,溶解的水铁矿再沉淀以结合游离的 Ni/Zn。然而,盐水入侵通过抑制非耐盐厌氧菌和铁还原菌的生长,强烈削弱了水铁矿的溶解-沉淀,并突出了伊利石-Ni/Zn的贡献。在微咸水侵入下,伊利石-锌对 Cb-Zn 的贡献增加了 12%,从而超过了 Fh-Zn (8%) 的贡献。在海水入侵下,几乎不发生水铁矿的溶解-沉淀,厌氧耐盐芽孢杆菌(> 95%) 占上风。因此,还原阶段 Cb-Ni/Zn (7%–15%) 的增加是由伊利石-Ni/Zn 贡献的。然而,在氧化阶段,碳酸盐取代了重新沉淀的水铁矿的最初作用,以结合溶液中的游离 Ni/Zn。这些新发现的机制可能是预测有毒元素的流动性和开发适当的盐分胁迫下可渗透反应屏障修复技术的关键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号