Synthetic Metals ( IF 4.0 ) Pub Date : 2022-06-22 , DOI: 10.1016/j.synthmet.2022.117102 Doğan Çirmi , Rukan Suna Karatekin , Rezzan Aydın , Fatih Köleli
|
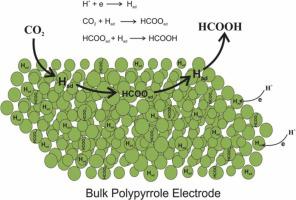
Conducting polymers are deposited mostly on a supporting metal of the Pt group and can be used as electrocatalysts. In the present work, a non-supported (bulk) polypyrrole (PPy) is prepared to eliminate any effect of the supporting metal on the desired reactions and is used for the hydrogen evolution reaction (HER) as well as for CO2 reduction in protic methanol for the first time. The physical, chemical, and electrochemical properties of the synthesized materials are studied in the first step. Scanning electron microscope images reveal that PPy particle size had a change in the range of 30–90 nm (conductivity: 6.2 ×10−2 ± 0.004 S/cm; four-probe). Tafel slope (82 mV/dec) and activation energy (41.67 kJ/mol) are calculated for HER using cyclic voltammograms. In the second step, the synthesized materials are used for the hydrogenation of CO2 in acidic methanol. The novel material reduces CO2 specifically to formic acid at −0.55 V with a maximum Faradaic efficiency of 92 %.
中文翻译:

非支撑聚吡咯电极上的氢析出和 CO2 还原
导电聚合物主要沉积在 Pt 族的支持金属上,可用作电催化剂。在目前的工作中,制备了一种非负载型(本体)聚吡咯(PPy)以消除负载金属对所需反应的任何影响,并用于析氢反应(HER)以及质子中的CO 2还原第一次用甲醇。第一步研究合成材料的物理、化学和电化学性质。扫描电镜图像显示,PPy 粒径在 30-90 nm 范围内变化(电导率:6.2 ×10 -2± 0.004 秒/厘米;四探头)。使用循环伏安图计算 HER 的 Tafel 斜率 (82 mV/dec) 和活化能 (41.67 kJ/mol)。第二步,合成的材料用于酸性甲醇中CO 2的加氢反应。这种新型材料在-0.55 V 时将CO 2专门还原为甲酸,最大法拉第效率为92%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号