Structure ( IF 4.4 ) Pub Date : 2022-06-22 , DOI: 10.1016/j.str.2022.05.021 Miles S Dickinson 1 , Takeshi Miyazawa 2 , Ryan S McCool 2 , Adrian T Keatinge-Clay 2
|
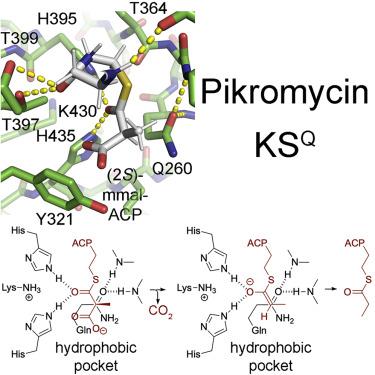
The first domain of modular polyketide synthases (PKSs) is most commonly a ketosynthase (KS)-like enzyme, KSQ, that primes polyketide synthesis. Unlike downstream KSs that fuse α-carboxyacyl groups to growing polyketide chains, it performs an extension-decoupled decarboxylation of these groups to generate primer units. When Pik127, a model triketide synthase constructed from modules of the pikromycin synthase, was studied by cryoelectron microscopy (cryo-EM), the dimeric didomain comprised of KSQ and the neighboring methylmalonyl-selective acyltransferase (AT) dominated the class averages and yielded structures at 2.5- and 2.8-Å resolution, respectively. Comparisons with ketosynthases complexed with their substrates revealed the conformation of the (2S)-methylmalonyl-S-phosphopantetheinyl portion of KSQ and KS substrates prior to decarboxylation. Point mutants of Pik127 probed the roles of residues in the KSQ active site, while an AT-swapped version of Pik127 demonstrated that KSQ can also decarboxylate malonyl groups. Mechanisms for how KSQ and KS domains catalyze carbon-carbon chemistry are proposed.
中文翻译:

来自匹克霉素合酶的启动酶揭示了装配线酮合酶如何催化碳碳化学
模块化聚酮合酶 (PKS) 的第一个结构域最常见的是类酮合酶 (KS) KS Q,它启动聚酮合酶的合成。与将 α-羧酰基融合到不断增长的聚酮化合物链的下游 KS 不同,它对这些基团进行延伸解耦脱羧以生成引物单元。当通过冷冻电子显微镜 (cryo-EM) 研究 Pik127(一种由匹克霉素合酶模块构建的模型三酮化合物合酶)时,由 KS Q 和邻近的甲基丙二酰选择性酰基转移酶 (AT) 组成的二聚体双结构域在类别平均值中占主导地位并产生了结构分辨率分别为 2.5 和 2.8 Å。与与其底物复合的酮合酶的比较揭示了脱羧之前KS Q和KS底物的( 2S )-甲基丙二酰基-S-磷酸泛酰胆碱部分的构象。Pik127 的点突变体探测了 KS Q活性位点中残基的作用,而 Pik127 的 AT 交换版本则证明 KS Q还可以使丙二酰基脱羧。提出了KS Q和 KS 结构域催化碳-碳化学的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号