Journal of Cleaner Production ( IF 9.7 ) Pub Date : 2022-06-25 , DOI: 10.1016/j.jclepro.2022.132819 Sibel Tunali Akar , Hilal Çolo , Fatih Sayin , Ilknur Kara , Tamer Akar
|
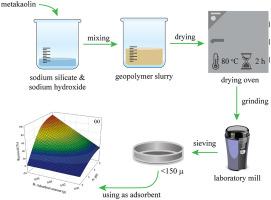
In the comprehensive research, the uptake of Cu(II) ions from contaminated water was explored using metakaolin-based geopolymer (MKG). The effects of contact time, adsorbent amount, initial pH, temperature, stirring speed, flow rate, column diameter, and adsorbate volume on the Cu(II) removal yield of MKG in the batch and column treatment systems were examined by employing Box-Behnken Design (BBD) approach. Cu(II) ions uptake was achieved at the optimum batch conditions of agitation speed: 160 rpm, temperature: ∼20 °C, initial pH: 5.3, contact time: 50 min, adsorbent amount: 0.04 g, with 95.02% removal yield. ANOVA results indicated that pH and adsorbent dosage were the most efficient Cu(II) adsorption parameters in the batch system. The PSO kinetic model could better describe the time-dependent data. Equilibrium adsorption data of Cu(II) showed a good adaptation with Langmuir isotherm with the maximum monolayer adsorption capacity of 1.36 × 10−3 mol g−1 (86.6 mg g−1). Breakthrough capacity at the exhausted point was computed as 42.2 mg g–1. The interaction mechanism between MKG and Cu(II) ions was tried to examine via zeta potential analysis, SEM/EDX, IR, XRD, and BET techniques. All the results have proven that MKG is a recoverable, cost-effective, and environment-friendly adsorbent for the removal of Cu(II) ions from aqueous solutions.
中文翻译:

偏高岭土基地质聚合物去除铜 (II) 工艺的参数优化:分批和连续工艺设计
在综合研究中,使用偏高岭土基地质聚合物 (MKG) 探索了从污染水中吸收 Cu(II) 离子。接触时间、吸附剂用量、初始 pH 值、温度、搅拌速度、流速、柱径的影响, 和吸附量对 MKG 在批处理和柱处理系统中的 Cu(II) 去除率的影响通过采用 Box-Behnken 设计 (BBD) 方法进行了检查。在搅拌速度:160 rpm,温度:~20 °C,初始 pH:5.3,接触时间:50 min,吸附剂用量:0.04 g,去除率 95.02% 的最佳批次条件下实现了 Cu(II) 离子的吸收。ANOVA 结果表明,pH 和吸附剂用量是批处理系统中最有效的 Cu(II) 吸附参数。PSO 动力学模型可以更好地描述时间相关数据。Cu(II) 的平衡吸附数据显示出与Langmuir 等温线的良好适应,最大单层吸附容量为 1.36 × 10 -3 mol g-1(86.6 mg g-1)。用尽点的突破能力计算为 42.2 mg g –1 。试图通过zeta电位分析、SEM/EDX、IR、XRD和BET技术检验MKG和Cu(II)离子之间的相互作用机制。所有结果都证明,MKG 是一种可回收、经济、环保的吸附剂,用于去除水溶液中的 Cu(II) 离子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号