当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral Counteranion-Directed Catalytic Asymmetric Methylene Migration Reaction of Ene-Aldimines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.joc.2c00742 Norie Momiyama 1, 2 , Chanantida Jongwohan 1, 2 , Naoya Ohtsuka 1, 2 , Pawittra Chaibuth 1, 3 , Takeshi Fujinami 1 , Kiyohiro Adachi 1, 4 , Toshiyasu Suzuki 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.joc.2c00742 Norie Momiyama 1, 2 , Chanantida Jongwohan 1, 2 , Naoya Ohtsuka 1, 2 , Pawittra Chaibuth 1, 3 , Takeshi Fujinami 1 , Kiyohiro Adachi 1, 4 , Toshiyasu Suzuki 1
Affiliation
|
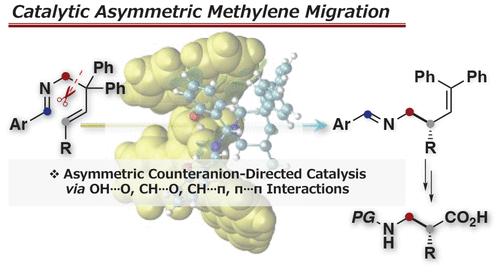
A catalytic asymmetric methylene migration reaction of ene-aldimines directed by chiral counteranions is developed, with the optimal catalyst identified as phenanthryl-substituted (R)-BINOL phosphate. Control experiments and density functional theory computations reveal the importance of the 2-hydroxy group of the ene-aldimine and attractive (e.g., OH···O, CH···O, CH···π, and π···π) interactions for high enantioselectivity (up to 74% ee). The results contribute to the design of asymmetric catalysis for the rearrangement of highly reactive iminium intermediates.
中文翻译:

烯醛亚胺的手性反阴离子导向催化不对称亚甲基迁移反应
开发了由手性抗衡阴离子引导的烯-醛亚胺催化不对称亚甲基迁移反应,最佳催化剂被确定为菲基取代的 ( R )-BINOL 磷酸盐。对照实验和密度泛函理论计算揭示了烯醛亚胺的 2-羟基的重要性和吸引力(例如 OH···O、CH···O、CH···π 和 π···π ) 相互作用以获得高对映选择性(高达 74% ee)。该结果有助于设计用于重排高反应性亚胺中间体的不对称催化。
更新日期:2022-06-23
中文翻译:

烯醛亚胺的手性反阴离子导向催化不对称亚甲基迁移反应
开发了由手性抗衡阴离子引导的烯-醛亚胺催化不对称亚甲基迁移反应,最佳催化剂被确定为菲基取代的 ( R )-BINOL 磷酸盐。对照实验和密度泛函理论计算揭示了烯醛亚胺的 2-羟基的重要性和吸引力(例如 OH···O、CH···O、CH···π 和 π···π ) 相互作用以获得高对映选择性(高达 74% ee)。该结果有助于设计用于重排高反应性亚胺中间体的不对称催化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号