当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Mechanistic Insights for the Oxygen Reduction Reaction in Chemically Modulated Ordered Intermetallic Catalyst Promoting Complete Electron Transfer
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-06-24 , DOI: 10.1021/jacs.2c04541 Soumi Mondal 1, 2 , Debabrata Bagchi 1, 2 , Mohd Riyaz 1, 2 , Shreya Sarkar 1, 2 , Ashutosh Kumar Singh 2, 3 , C P Vinod 4 , Sebastian C Peter 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-06-24 , DOI: 10.1021/jacs.2c04541 Soumi Mondal 1, 2 , Debabrata Bagchi 1, 2 , Mohd Riyaz 1, 2 , Shreya Sarkar 1, 2 , Ashutosh Kumar Singh 2, 3 , C P Vinod 4 , Sebastian C Peter 1, 2
Affiliation
|
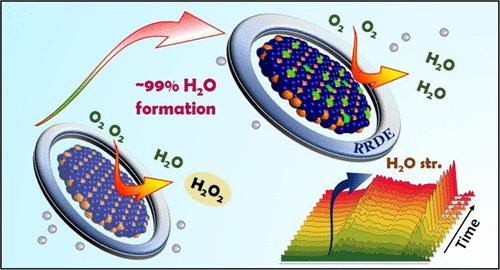
The well-known limitation of alkaline fuel cells is the slack kinetics of the cathodic half-cell reaction, the oxygen reduction reaction (ORR). Platinum, being the most active ORR catalyst, is still facing challenges due to its corrosive nature and sluggish kinetics. Many novel approaches for substituting Pt have been reported, which suffer from stability issues even after mighty modifications. Designing an extremely stable, but unexplored ordered intermetallic structure, Pd2Ge, and tuning the electronic environment of the active sites by site-selective Pt substitution to overcome the hurdle of alkaline ORR is the main motive of this paper. The substitution of platinum atoms at a specific Pd position leads to Pt0.2Pd1.8Ge demonstrating a half-wave potential (E1/2) of 0.95 V vs RHE, which outperforms the state-of-the-art catalyst 20% Pt/C. The mass activity (MA) of Pt0.2Pd1.8Ge is 320 mA/mgPt, which is almost 3.2 times better than that of Pt/C. E1/2 and MA remained unaltered even after 50,000 accelerated degradation test (ADT) cycles, which makes it a promising stable catalyst with its activity better than that of the state-of-the-art Pt/C. The undesired 2e– transfer ORR forming hydrogen peroxide (H2O2) is diminished in Pt0.2Pd1.8Ge as visible from the rotating ring-disk electrode (RRDE) experiment, spectroscopically visualized by in situ Fourier transform infrared (FTIR) spectroscopy and supported by computational studies. The effect of Pt substitution on Pd has been properly manifested by X-ray absorption spectroscopy (XAS) and X-ray photoelectron spectroscopy (XPS). The swinging of the oxidation state of atomic sites of Pt0.2Pd1.8Ge during the reaction is probed by in situ XAS, which efficiently enhances 4e– transfer, producing an extremely low percentage of H2O2.
中文翻译:

化学调制有序金属间化合物催化剂中氧还原反应促进电子完全转移的原位机理研究
众所周知,碱性燃料电池的限制是阴极半电池反应的松弛动力学,即氧还原反应 (ORR)。铂作为最活跃的 ORR 催化剂,由于其腐蚀性和缓慢的动力学,仍然面临挑战。已经报道了许多替代 Pt 的新方法,即使经过强大的修改,它们也存在稳定性问题。设计一种极其稳定但尚未探索的有序金属间化合物结构 Pd 2 Ge,并通过位点选择性 Pt 替代调整活性位点的电子环境以克服碱性 ORR 的障碍是本文的主要动机。铂原子在特定 Pd 位置的取代导致 Pt 0.2 Pd 1.8 Ge 表现出半波电位(E 1/2 ) 为 0.95 V vs RHE,其性能优于最先进的催化剂 20% Pt/C。Pt 0.2 Pd 1.8 Ge的质量活度(MA)为320 mA/mg Pt,几乎是Pt/C的3.2倍。即使经过 50,000 次加速降解测试 (ADT) 循环, E 1/2和 MA 仍然保持不变,这使其成为一种很有前途的稳定催化剂,其活性优于最先进的 Pt/C。在 Pt 0.2 Pd 1.8中减少了不希望的 2e –转移 ORR 形成过氧化氢 (H 2 O 2 )Ge 从旋转环盘电极 (RRDE) 实验中可见,通过原位傅里叶变换红外 (FTIR) 光谱进行光谱可视化,并得到计算研究的支持。Pt 取代对 Pd 的影响已通过 X 射线吸收光谱 (XAS) 和 X 射线光电子能谱 (XPS) 适当地表现出来。原位 XAS 探测了反应过程中 Pt 0.2 Pd 1.8 Ge原子位点氧化态的摆动,有效地增强了 4e 转移,产生了极低百分比的 H 2 O 2。
更新日期:2022-06-24
中文翻译:

化学调制有序金属间化合物催化剂中氧还原反应促进电子完全转移的原位机理研究
众所周知,碱性燃料电池的限制是阴极半电池反应的松弛动力学,即氧还原反应 (ORR)。铂作为最活跃的 ORR 催化剂,由于其腐蚀性和缓慢的动力学,仍然面临挑战。已经报道了许多替代 Pt 的新方法,即使经过强大的修改,它们也存在稳定性问题。设计一种极其稳定但尚未探索的有序金属间化合物结构 Pd 2 Ge,并通过位点选择性 Pt 替代调整活性位点的电子环境以克服碱性 ORR 的障碍是本文的主要动机。铂原子在特定 Pd 位置的取代导致 Pt 0.2 Pd 1.8 Ge 表现出半波电位(E 1/2 ) 为 0.95 V vs RHE,其性能优于最先进的催化剂 20% Pt/C。Pt 0.2 Pd 1.8 Ge的质量活度(MA)为320 mA/mg Pt,几乎是Pt/C的3.2倍。即使经过 50,000 次加速降解测试 (ADT) 循环, E 1/2和 MA 仍然保持不变,这使其成为一种很有前途的稳定催化剂,其活性优于最先进的 Pt/C。在 Pt 0.2 Pd 1.8中减少了不希望的 2e –转移 ORR 形成过氧化氢 (H 2 O 2 )Ge 从旋转环盘电极 (RRDE) 实验中可见,通过原位傅里叶变换红外 (FTIR) 光谱进行光谱可视化,并得到计算研究的支持。Pt 取代对 Pd 的影响已通过 X 射线吸收光谱 (XAS) 和 X 射线光电子能谱 (XPS) 适当地表现出来。原位 XAS 探测了反应过程中 Pt 0.2 Pd 1.8 Ge原子位点氧化态的摆动,有效地增强了 4e 转移,产生了极低百分比的 H 2 O 2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号