当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Absolute and Relative Positioning of Natural Organic Matter Acid–Base Potentiometric Titration Curves: Implications for the Evaluation of the Density of Charged Reactive Sites
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.est.2c00828 Marawit Tesfa 1 , Jérôme F L Duval 2 , Rémi Marsac 1 , Aline Dia 1 , Jose-Paulo Pinheiro 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.est.2c00828 Marawit Tesfa 1 , Jérôme F L Duval 2 , Rémi Marsac 1 , Aline Dia 1 , Jose-Paulo Pinheiro 2
Affiliation
|
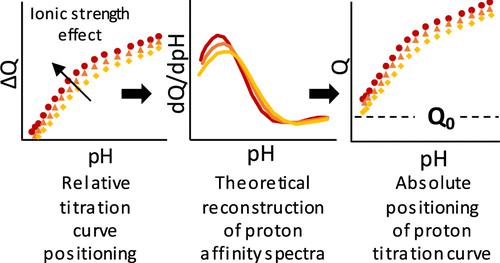
Potentiometric acid–base titration curves collected on humic (nano)particles as a function of pH and salt concentration reflect the electrostatics of the particles and the amount of chemical charges (Q) they carry. In turn, the interpretation of titration data helps quantify their reactivity toward metals provided that both intrinsic chemical and nonspecific electrostatic contributions to proton binding are correctly unraveled. Establishing a titration curve requires several steps, i.e., blank subtraction, relative curve positioning with respect to the electrolyte concentration, and absolute curve positioning achieved by the estimation of particle charge Q0 at low pH. Failure to properly establish each step may lead to the misevaluation of nanoparticle charging behavior. Here, we report (i) a simple procedure to measure and position titration curves for humic substances (HS) versus salt concentration and (ii) an original approach for absolute curve positioning upon the exploitation of proton affinity spectra. The latter do not depend on Q0 and they thus constrain the titration data analysis using the soft Poisson–Boltzmann-based titration (SPBT) formalism for nanoparticles in the thick electric double-layer regime. We illustrate the benefits of our approach by analyzing titration measurements for a large range of humic nanoparticles and by comparing the outcome with results from the literature.
中文翻译:

天然有机物质酸碱电位滴定曲线的绝对和相对定位:对带电反应位点密度评估的意义
在腐殖质(纳米)颗粒上收集的作为 pH 值和盐浓度函数的电位酸碱滴定曲线反映了颗粒的静电和它们携带的化学电荷量 ( Q )。反过来,只要正确解开对质子结合的内在化学和非特异性静电贡献,对滴定数据的解释有助于量化它们对金属的反应性。建立滴定曲线需要几个步骤,即空白减去,相对于电解质浓度的相对曲线定位,以及通过估计粒子电荷Q 0实现的绝对曲线定位在低 pH 值下。未能正确建立每个步骤可能会导致对纳米粒子充电行为的错误评估。在这里,我们报告 (i) 测量和定位腐殖质 (HS) 与盐浓度的滴定曲线的简单程序,以及 (ii) 利用质子亲和光谱进行绝对曲线定位的原始方法。后者不依赖于Q 0,因此它们限制了使用基于软泊松-玻尔兹曼滴定 (SPBT) 形式对厚电双层体系中的纳米粒子进行的滴定数据分析。我们通过分析大量腐殖质纳米颗粒的滴定测量并将结果与文献结果进行比较来说明我们方法的好处。
更新日期:2022-06-24
中文翻译:

天然有机物质酸碱电位滴定曲线的绝对和相对定位:对带电反应位点密度评估的意义
在腐殖质(纳米)颗粒上收集的作为 pH 值和盐浓度函数的电位酸碱滴定曲线反映了颗粒的静电和它们携带的化学电荷量 ( Q )。反过来,只要正确解开对质子结合的内在化学和非特异性静电贡献,对滴定数据的解释有助于量化它们对金属的反应性。建立滴定曲线需要几个步骤,即空白减去,相对于电解质浓度的相对曲线定位,以及通过估计粒子电荷Q 0实现的绝对曲线定位在低 pH 值下。未能正确建立每个步骤可能会导致对纳米粒子充电行为的错误评估。在这里,我们报告 (i) 测量和定位腐殖质 (HS) 与盐浓度的滴定曲线的简单程序,以及 (ii) 利用质子亲和光谱进行绝对曲线定位的原始方法。后者不依赖于Q 0,因此它们限制了使用基于软泊松-玻尔兹曼滴定 (SPBT) 形式对厚电双层体系中的纳米粒子进行的滴定数据分析。我们通过分析大量腐殖质纳米颗粒的滴定测量并将结果与文献结果进行比较来说明我们方法的好处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号