当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chromium Nitride Umpolung Tuned by the Locus of Oxidation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-06-24 , DOI: 10.1021/jacs.2c01840 Diego Martelino 1 , Samyadeb Mahato 1 , Warren VandeVen 1 , Nicholas M Hein 1 , Ryan M Clarke 1 , Gregory A MacNeil 1 , Fabrice Thomas 2 , Tim Storr 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-06-24 , DOI: 10.1021/jacs.2c01840 Diego Martelino 1 , Samyadeb Mahato 1 , Warren VandeVen 1 , Nicholas M Hein 1 , Ryan M Clarke 1 , Gregory A MacNeil 1 , Fabrice Thomas 2 , Tim Storr 1
Affiliation
|
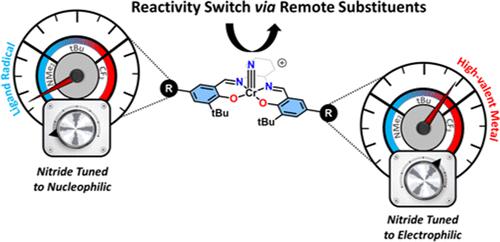
Oxidation of a series of CrV nitride salen complexes (CrVNSalR) with different para-phenolate substituents (R = CF3, tBu, NMe2) was investigated to determine how the locus of oxidation (either metal or ligand) dictates reactivity at the nitride. Para-phenolate substituents were chosen to provide maximum variation in the electron-donating ability of the tetradentate ligand at a site remote from the metal coordination sphere. We show that one-electron oxidation affords CrVI nitrides ([CrVINSalR]+; R = CF3, tBu) and a localized CrV nitride phenoxyl radical for the more electron-donating NMe2 substituent ([CrVNSalNMe2]•+). The facile nitride homocoupling observed for the MnVI analogues was significantly attenuated for the CrVI complexes due to a smaller increase in nitride character in the M≡N π* orbitals for Cr relative to Mn. Upon oxidation, both the calculated nitride natural population analysis (NPA) charge and energy of molecular orbitals associated with the {Cr≡N} unit change to a lesser extent for the CrV ligand radical derivative ([CrVNSalNMe2]•+) in comparison to the CrVI derivatives ([CrVINSalR]+; R = CF3, tBu). As a result, [CrVNSalNMe2]•+ reacts with B(C6F5)3, thus exhibiting similar nucleophilic reactivity to the neutral CrV nitride derivatives. In contrast, the CrVI derivatives ([CrVINSalR]+; R = CF3, tBu) act as electrophiles, displaying facile reactivity with PPh3 and no reaction with B(C6F5)3. Thus, while oxidation to the ligand radical does not change the reactivity profile, metal-based oxidation to CrVI results in umpolung, a switch from nucleophilic to electrophilic reactivity at the terminal nitride.
中文翻译:

由氧化位点调谐的氮化铬 Umpolung
研究了一系列具有不同对苯酚取代基 (R = CF 3 , tBu, NMe 2 ) 的 Cr V氮化物salen配合物( Cr V NSal R )的氧化,以确定氧化位点(金属或配体)如何决定反应性在氮化物处。选择对苯酚取代基以在远离金属配位球的位置提供四齿配体的给电子能力的最大变化。我们表明,单电子氧化产生 Cr VI氮化物 ( [Cr VI NSal R ] + ; R = CF 3, tBu) 和一个局部的 Cr V氮化物苯氧基自由基,用于更多的给电子 NMe 2取代基 ( [Cr V NSal NMe2 ] •+ )。由于相对于 Mn,Cr 在 M≡N π* 轨道中氮化物特性的增加较小,因此对 Mn VI类似物观察到的氮化物同源耦合显着减弱了 Cr VI配合物。氧化后,与 {Cr≡N} 单元相关的分子轨道计算的氮化物自然种群分析 (NPA) 电荷和能量对于 Cr V配体自由基衍生物 ( [Cr V NSal NMe2 ]•+ ) 与 Cr VI衍生物 ( [Cr VI NSal R ] + ; R = CF 3 , tBu) 相比。结果, [Cr V NSal NMe2 ] •+与B(C 6 F 5 ) 3反应,因此表现出与中性Cr V氮化物衍生物相似的亲核反应性相比之下,Cr VI衍生物 ( [Cr VI NSal R ] + ; R = CF 3 , tBu) 充当亲电体,与 PPh 3表现出容易的反应性并且不与B(C 6 F 5 ) 3反应。因此,虽然氧化成配体自由基不会改变反应性分布,但金属基氧化成 Cr VI会导致 umpolung,即在末端氮化物处从亲核反应性转变为亲电性反应性。
更新日期:2022-06-24
中文翻译:

由氧化位点调谐的氮化铬 Umpolung
研究了一系列具有不同对苯酚取代基 (R = CF 3 , tBu, NMe 2 ) 的 Cr V氮化物salen配合物( Cr V NSal R )的氧化,以确定氧化位点(金属或配体)如何决定反应性在氮化物处。选择对苯酚取代基以在远离金属配位球的位置提供四齿配体的给电子能力的最大变化。我们表明,单电子氧化产生 Cr VI氮化物 ( [Cr VI NSal R ] + ; R = CF 3, tBu) 和一个局部的 Cr V氮化物苯氧基自由基,用于更多的给电子 NMe 2取代基 ( [Cr V NSal NMe2 ] •+ )。由于相对于 Mn,Cr 在 M≡N π* 轨道中氮化物特性的增加较小,因此对 Mn VI类似物观察到的氮化物同源耦合显着减弱了 Cr VI配合物。氧化后,与 {Cr≡N} 单元相关的分子轨道计算的氮化物自然种群分析 (NPA) 电荷和能量对于 Cr V配体自由基衍生物 ( [Cr V NSal NMe2 ]•+ ) 与 Cr VI衍生物 ( [Cr VI NSal R ] + ; R = CF 3 , tBu) 相比。结果, [Cr V NSal NMe2 ] •+与B(C 6 F 5 ) 3反应,因此表现出与中性Cr V氮化物衍生物相似的亲核反应性相比之下,Cr VI衍生物 ( [Cr VI NSal R ] + ; R = CF 3 , tBu) 充当亲电体,与 PPh 3表现出容易的反应性并且不与B(C 6 F 5 ) 3反应。因此,虽然氧化成配体自由基不会改变反应性分布,但金属基氧化成 Cr VI会导致 umpolung,即在末端氮化物处从亲核反应性转变为亲电性反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号