当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Water-Soluble Planar Cobalt(II), Nickel(II), and Copper(II) Hydroxo Clusters Using a (1,4,7-Triazacyclononane)cobalt(III) Complex as a Hydrolysis-Terminating Group
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.inorgchem.2c01046 Sugiarto 1 , Yuya Imai 1 , Yoshihito Hayashi 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.inorgchem.2c01046 Sugiarto 1 , Yuya Imai 1 , Yoshihito Hayashi 1
Affiliation
|
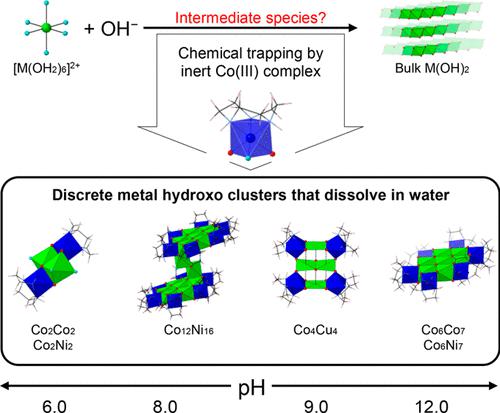
We report on a group of planar cobalt(II), nickel(II), and copper(II) hydroxo clusters that have a definite composition and are water-soluble: [{Co(tacn)(OH)2}6Co7(OH)12](NO3)2(CF3SO3)6·10H2O (1), [{Co(tacn)(OH)2}6Ni7(OH)12](NO3)2(CF3SO3)6·10H2O (2a), [{Co(tacn)(OH)2}6Ni7(OH)12](BNPP)8·6CH3NO2·8H2O [2b; BNPP = bis(p-nitrophenyl)phosphate], [{Co(tacn)(OH)2}12Ni16(OH)26(OH2)2](SO4)4(CF3SO3)10·30H2O (3a), [{Co(tacn)(OH)2}12Ni16(OH)26(OH2)2](SO4)8(CF3SO3)2·44H2O (3b), [{Co(tacn)(OH)2}2Co2(OH)2(OH2)4](SO4)(CF3SO3)2·4H2O (4), [{Co(tacn)(OH)2}2Ni2(OH)2(OH2)4](SO4)(CF3SO3)2·4H2O (5), and [{Co(tacn)(OH)2}4Cu4(OH)6](ClO4)6·5H2O (6), where tacn is 1,4,7-triazacyclononane. The peripheral of each metal hydroxo cluster plane is chemically protected by the coordination of {CoIII(tacn)(OH)2}+ groups to prevent further hydrolysis. These clusters were synthesized by the reaction of an equimolar amount of [Co(tacn)(OH2)3]3+ and cobalt, nickel, or copper salt at pH values in the range of 6.0–12.0. The structure of the cation in compounds 1, 2a or 2b, 4, and 5 is relevant to the surface structure of the cobalt phosphate and nickel borate oxygen-evolution catalysts; in particular, the Co7(OH)12 core in 1. Moreover, the arrangement of M7(OH)12 in 1 and 2a or 2b and Cu4(OH)6 in 6 represents the solid-state structures of the (111) face of the cubic CoO or NiO and the (002) plane of Cu(OH)2, respectively. Extended X-ray absorption fine structure spectra of an aqueous solution of 1, 2a, 4, and 5 exhibit well-resolved peaks at the first and second coordination spheres due to the M–O and M···M distances, respectively; the solution-state bond distances were estimated, and they agreed well with the bond distances in the solid-state structures.
中文翻译:

使用 (1,4,7-三氮杂环壬烷) 钴 (III) 配合物作为水解终止基团合成水溶性平面钴 (II)、镍 (II) 和铜 (II) 羟基簇
我们报告了一组平面钴 (II)、镍 (II) 和铜 (II) 羟基簇,它们具有确定的组成并且是水溶性的:[{Co(tacn)(OH) 2 } 6 Co 7 ( OH) 12 ](NO 3 ) 2 (CF 3 SO 3 ) 6 ·10H 2 O ( 1 ), [{Co(tacn)(OH) 2 } 6 Ni 7 (OH) 12 ](NO 3 ) 2 (CF 3 SO 3 ) 6 ·10H 2 O ( 2a ), [{Co(tacn)(OH)2 } 6 Ni 7 (OH) 12 ](BNPP) 8 ·6CH 3 NO 2 ·8H 2 O [ 2b ; BNPP = 双(对硝基苯基)磷酸盐], [{Co(tacn)(OH) 2 } 12 Ni 16 (OH) 26 (OH 2 ) 2 ](SO 4 ) 4 (CF 3 SO 3 ) 10 ·30H 2 O ( 3a ), [{Co(tacn)(OH) 2 } 12 Ni 16 (OH) 26(OH 2 ) 2 ](SO 4 ) 8 (CF 3 SO 3 ) 2 ·44H 2 O ( 3b ), [{Co(tacn)(OH) 2 } 2 Co 2 (OH) 2 (OH 2 ) 4 ] (SO 4 )(CF 3 SO 3 ) 2 ·4H 2 O ( 4 ), [{Co(tacn)(OH) 2 } 2 Ni 2 (OH) 2 (OH 2 ) 4 ](SO 4)(CF 3 SO 3 ) 2 ·4H 2 O ( 5 ),和[{Co(tacn)(OH) 2 } 4 Cu 4 (OH) 6 ](ClO 4 ) 6 ·5H 2 O ( 6 ),其中tacn 是 1,4,7-三氮杂环壬烷。每个金属羟基簇平面的外围通过{Co III (tacn)(OH) 2 } +基团的配位化学保护以防止进一步水解。这些簇是由等摩尔量的[Co(tacn)(OH 2 ) 3 ] 3+反应合成的pH 值在 6.0–12.0 范围内的钴、镍或铜盐。化合物1、2a或2b、4、5中阳离子的结构与磷酸钴和硼酸镍析氧催化剂的表面结构有关;特别是1中的 Co 7 (OH) 12核。此外,M 7 (OH) 12在1和2a或2b中的排列和 Cu 4 (OH) 6在6中的排列分别表示立方CoO或NiO的(111)面和Cu(OH) 2的(002)面的固态结构。由于 M-O 和 M···M 距离,1、2a、4 和 5 的水溶液的扩展 X 射线吸收精细结构光谱在第一和第二配位层处表现出良好分辨的峰;估计了溶液态键距,它们与固态结构中的键距非常吻合。
更新日期:2022-06-24
中文翻译:

使用 (1,4,7-三氮杂环壬烷) 钴 (III) 配合物作为水解终止基团合成水溶性平面钴 (II)、镍 (II) 和铜 (II) 羟基簇
我们报告了一组平面钴 (II)、镍 (II) 和铜 (II) 羟基簇,它们具有确定的组成并且是水溶性的:[{Co(tacn)(OH) 2 } 6 Co 7 ( OH) 12 ](NO 3 ) 2 (CF 3 SO 3 ) 6 ·10H 2 O ( 1 ), [{Co(tacn)(OH) 2 } 6 Ni 7 (OH) 12 ](NO 3 ) 2 (CF 3 SO 3 ) 6 ·10H 2 O ( 2a ), [{Co(tacn)(OH)2 } 6 Ni 7 (OH) 12 ](BNPP) 8 ·6CH 3 NO 2 ·8H 2 O [ 2b ; BNPP = 双(对硝基苯基)磷酸盐], [{Co(tacn)(OH) 2 } 12 Ni 16 (OH) 26 (OH 2 ) 2 ](SO 4 ) 4 (CF 3 SO 3 ) 10 ·30H 2 O ( 3a ), [{Co(tacn)(OH) 2 } 12 Ni 16 (OH) 26(OH 2 ) 2 ](SO 4 ) 8 (CF 3 SO 3 ) 2 ·44H 2 O ( 3b ), [{Co(tacn)(OH) 2 } 2 Co 2 (OH) 2 (OH 2 ) 4 ] (SO 4 )(CF 3 SO 3 ) 2 ·4H 2 O ( 4 ), [{Co(tacn)(OH) 2 } 2 Ni 2 (OH) 2 (OH 2 ) 4 ](SO 4)(CF 3 SO 3 ) 2 ·4H 2 O ( 5 ),和[{Co(tacn)(OH) 2 } 4 Cu 4 (OH) 6 ](ClO 4 ) 6 ·5H 2 O ( 6 ),其中tacn 是 1,4,7-三氮杂环壬烷。每个金属羟基簇平面的外围通过{Co III (tacn)(OH) 2 } +基团的配位化学保护以防止进一步水解。这些簇是由等摩尔量的[Co(tacn)(OH 2 ) 3 ] 3+反应合成的pH 值在 6.0–12.0 范围内的钴、镍或铜盐。化合物1、2a或2b、4、5中阳离子的结构与磷酸钴和硼酸镍析氧催化剂的表面结构有关;特别是1中的 Co 7 (OH) 12核。此外,M 7 (OH) 12在1和2a或2b中的排列和 Cu 4 (OH) 6在6中的排列分别表示立方CoO或NiO的(111)面和Cu(OH) 2的(002)面的固态结构。由于 M-O 和 M···M 距离,1、2a、4 和 5 的水溶液的扩展 X 射线吸收精细结构光谱在第一和第二配位层处表现出良好分辨的峰;估计了溶液态键距,它们与固态结构中的键距非常吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号