当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper Substitution in P2-Type Sodium Layered Oxide To Mitigate Phase Transition and Enhance Cyclability of Sodium-Ion Batteries
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-06-24 , DOI: 10.1021/acsami.2c05521 Yanfen Wen 1, 2 , Zheng Huang 2 , Jiabo Le 3 , Peng Dai 2 , Chenguang Shi 2 , Gen Li 2 , Shiyuan Zhou 2 , Jingjing Fan 2 , Shuxin Zhuang 1 , Mi Lu 1 , Ling Huang 2 , Shi-Gang Sun 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-06-24 , DOI: 10.1021/acsami.2c05521 Yanfen Wen 1, 2 , Zheng Huang 2 , Jiabo Le 3 , Peng Dai 2 , Chenguang Shi 2 , Gen Li 2 , Shiyuan Zhou 2 , Jingjing Fan 2 , Shuxin Zhuang 1 , Mi Lu 1 , Ling Huang 2 , Shi-Gang Sun 2
Affiliation
|
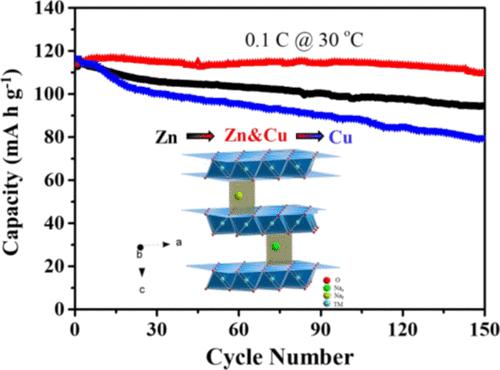
Development of high-performance cathode materials is one of the key challenges in the practical application of sodium-ion batteries. Among all the cathode materials, layered sodium transition-metal oxides are particularly attractive. However, undesired phase transitions are often reported and have detrimental effects on the structure stability and electrochemical performance. Cu substitution of zinc in the P2-type Na0.6Mn0.7Ni0.15Zn0.15–xCuxO2 (x = 0, 0.075, and 0.15) composites was investigated in this study for mitigating the biphase transition and enhancing the electrochemical performance of sodium-ion batteries. The coupling effect of Zn and Cu enables an excellent capacity retention of 96.4% of the initial discharge capacity after 150 cycles at 0.1 C in the Na/Na0.6Mn0.7Ni0.15Zn0.075Cu0.075O2 cell. The biphase transition that occurred in the high voltage range has been significantly suppressed after the incorporation of Cu in Na0.6Mn0.7Ni0.15Zn0.15O2, which was confirmed by in situ X-ray diffraction studies. Moreover, the substitution of the inert element Zn with electrochemically active Cu leads to the suppression of anionic redox and the occurrence of Cu2+/3+ redox reaction, and the electrolyte decomposition is impeded after the introduction of electrochemically active Cu in the Na0.6Mn0.7Ni0.15Zn0.15–xCuxO2 composite cathode. The enhanced electrochemical performance in the Na0.6Mn0.7Ni0.15Zn0.075Cu0.075O2 electrode can be ascribed to the coexistence of Zn and Cu and alleviated volumetric change as well as suppressed electrode/electrolyte side reaction after Cu substitution.
中文翻译:

P2型层状氧化钠中的铜取代以减轻相变并增强钠离子电池的可循环性
高性能正极材料的开发是钠离子电池实际应用的关键挑战之一。在所有正极材料中,层状钠过渡金属氧化物尤其具有吸引力。然而,不希望的相变经常被报道并且对结构稳定性和电化学性能具有不利影响。P2型中锌的Cu置换 Na 0.6 Mn 0.7 Ni 0.15 Zn 0.15– x Cu x O 2 ( x= 0、0.075 和 0.15) 复合材料在本研究中进行了研究,以减轻双相转变并提高钠离子电池的电化学性能。Zn 和 Cu 的耦合效应使 Na/Na 0.6 Mn 0.7 Ni 0.15 Zn 0.075 Cu 0.075 O 2电池在 0.1 C 下 150 次循环后具有出色的容量保持率,达到初始放电容量的 96.4%。在Na 0.6 Mn 0.7 Ni 0.15 Zn 0.15 O 2中掺入Cu后,在高压范围内发生的双相转变得到了显着抑制, 原位 X 射线衍射研究证实了这一点。此外,惰性元素Zn被电化学活性Cu取代导致阴离子氧化还原受到抑制,Cu 2+/3+氧化还原反应发生,在Na 0.6中引入电化学活性Cu后电解液分解受到阻碍。Mn 0.7 Ni 0.15 Zn 0.15– x Cu x O 2复合正极。Na 0.6 Mn 0.7 Ni 0.15 Zn 0.075 Cu 0.075 O 2中增强的电化学性能电极可归因于 Zn 和 Cu 的共存,减轻了体积变化,并抑制了 Cu 取代后的电极/电解质副反应。
更新日期:2022-06-24
中文翻译:

P2型层状氧化钠中的铜取代以减轻相变并增强钠离子电池的可循环性
高性能正极材料的开发是钠离子电池实际应用的关键挑战之一。在所有正极材料中,层状钠过渡金属氧化物尤其具有吸引力。然而,不希望的相变经常被报道并且对结构稳定性和电化学性能具有不利影响。P2型中锌的Cu置换 Na 0.6 Mn 0.7 Ni 0.15 Zn 0.15– x Cu x O 2 ( x= 0、0.075 和 0.15) 复合材料在本研究中进行了研究,以减轻双相转变并提高钠离子电池的电化学性能。Zn 和 Cu 的耦合效应使 Na/Na 0.6 Mn 0.7 Ni 0.15 Zn 0.075 Cu 0.075 O 2电池在 0.1 C 下 150 次循环后具有出色的容量保持率,达到初始放电容量的 96.4%。在Na 0.6 Mn 0.7 Ni 0.15 Zn 0.15 O 2中掺入Cu后,在高压范围内发生的双相转变得到了显着抑制, 原位 X 射线衍射研究证实了这一点。此外,惰性元素Zn被电化学活性Cu取代导致阴离子氧化还原受到抑制,Cu 2+/3+氧化还原反应发生,在Na 0.6中引入电化学活性Cu后电解液分解受到阻碍。Mn 0.7 Ni 0.15 Zn 0.15– x Cu x O 2复合正极。Na 0.6 Mn 0.7 Ni 0.15 Zn 0.075 Cu 0.075 O 2中增强的电化学性能电极可归因于 Zn 和 Cu 的共存,减轻了体积变化,并抑制了 Cu 取代后的电极/电解质副反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号