Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elastic Nanovaccine Enhances Dendritic Cell-Mediated Tumor Immunotherapy
Small ( IF 13.0 ) Pub Date : 2022-06-22 , DOI: 10.1002/smll.202201108 Qiang Li 1 , Zhaogang Teng 2 , Jun Tao 2 , Wenhui Shi 2 , Guangwen Yang 1 , Yu Zhang 1 , Xiaodan Su 2 , Lin Chen 1 , Weijun Xiu 2 , Lihui Yuwen 2 , Heng Dong 1 , Yongbin Mou 1
Small ( IF 13.0 ) Pub Date : 2022-06-22 , DOI: 10.1002/smll.202201108 Qiang Li 1 , Zhaogang Teng 2 , Jun Tao 2 , Wenhui Shi 2 , Guangwen Yang 1 , Yu Zhang 1 , Xiaodan Su 2 , Lin Chen 1 , Weijun Xiu 2 , Lihui Yuwen 2 , Heng Dong 1 , Yongbin Mou 1
Affiliation
|
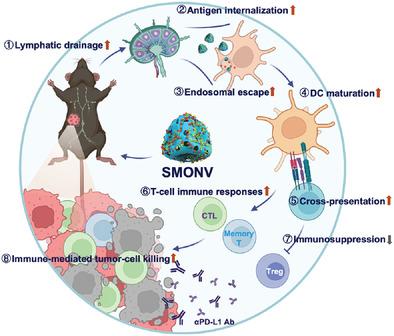
Nanovaccine-based immunotherapy (NBI) has the ability to initiate dendritic cell (DC)-mediated tumor-specific immune responses and maintain long-term antitumor immune memory. To date, the mechanism by which the mechanical properties of nanoparticles alter the functions of DCs in NBI remains largely unclear. Here, a soft mesoporous organosilica-based nanovaccine (SMONV) is prepared and the elasticity-dependent effect of the nanovaccine on the underlying DC-mediated immune responses is studied. It is found that the elasticity results in greater internalization of SMONV by DCs, followed by the induction of substantial cytosolic delivery of antigens via endosomal escape, leading to effective DC maturation and antigen cross-presentation. Impressively, elasticity enables SMONV to enhance lymphatic drainage of antigens in vivo, thus stimulating robust humoral and cellular immunity. The results from therapeutic tumor vaccination further reveal that subcutaneously administered SMONV effectively suppresses tumor growth in tumor-bearing mice by evoking antigen-specific CD8+ T-cell immune responses, mitigating regulatory T-cell-mediated immunosuppression, and increasing central memory and effector memory T-cell populations. Furthermore, combinatorial immunization with SMONV and anti-PD-L1 blocking antibodies results in an amplified therapeutic effect on tumor-bearing mice. These findings reveal the elastic effect of the nanovaccine on DC-mediated immune responses, and the prepared SMONV represents a facile and powerful strategy for antitumor immunotherapy.
中文翻译:

弹性纳米疫苗增强树突状细胞介导的肿瘤免疫治疗
基于纳米疫苗的免疫疗法 (NBI) 具有启动树突状细胞 (DC) 介导的肿瘤特异性免疫反应并维持长期抗肿瘤免疫记忆的能力。迄今为止,纳米粒子的机械性能改变 NBI 中 DCs 功能的机制仍不清楚。在这里,制备了一种基于软介孔有机硅的纳米疫苗(SMONV),并研究了纳米疫苗对潜在 DC 介导的免疫反应的弹性依赖性影响。发现弹性导致 DCs 对 SMONV 的更大内化,然后通过内体逃逸诱导大量细胞溶质递送抗原,导致有效的 DC 成熟和抗原交叉呈递。令人印象深刻的是,弹性使 SMONV 能够增强体内抗原的淋巴引流,从而刺激强大的体液和细胞免疫。治疗性肿瘤疫苗接种的结果进一步表明,皮下注射 SMONV 通过诱发抗原特异性 CD8 有效抑制荷瘤小鼠的肿瘤生长+ T 细胞免疫反应,减轻调节性 T 细胞介导的免疫抑制,并增加中枢记忆和效应记忆 T 细胞群。此外,使用 SMONV 和抗 PD-L1 阻断抗体进行联合免疫可增强对荷瘤小鼠的治疗效果。这些发现揭示了纳米疫苗对 DC 介导的免疫反应的弹性作用,制备的 SMONV 代表了一种简便而有效的抗肿瘤免疫治疗策略。
更新日期:2022-06-22
中文翻译:

弹性纳米疫苗增强树突状细胞介导的肿瘤免疫治疗
基于纳米疫苗的免疫疗法 (NBI) 具有启动树突状细胞 (DC) 介导的肿瘤特异性免疫反应并维持长期抗肿瘤免疫记忆的能力。迄今为止,纳米粒子的机械性能改变 NBI 中 DCs 功能的机制仍不清楚。在这里,制备了一种基于软介孔有机硅的纳米疫苗(SMONV),并研究了纳米疫苗对潜在 DC 介导的免疫反应的弹性依赖性影响。发现弹性导致 DCs 对 SMONV 的更大内化,然后通过内体逃逸诱导大量细胞溶质递送抗原,导致有效的 DC 成熟和抗原交叉呈递。令人印象深刻的是,弹性使 SMONV 能够增强体内抗原的淋巴引流,从而刺激强大的体液和细胞免疫。治疗性肿瘤疫苗接种的结果进一步表明,皮下注射 SMONV 通过诱发抗原特异性 CD8 有效抑制荷瘤小鼠的肿瘤生长+ T 细胞免疫反应,减轻调节性 T 细胞介导的免疫抑制,并增加中枢记忆和效应记忆 T 细胞群。此外,使用 SMONV 和抗 PD-L1 阻断抗体进行联合免疫可增强对荷瘤小鼠的治疗效果。这些发现揭示了纳米疫苗对 DC 介导的免疫反应的弹性作用,制备的 SMONV 代表了一种简便而有效的抗肿瘤免疫治疗策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号