当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, solid-state, solution, and theoretical characterization of an “in-cage” scandium-NOTA complex
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-06-24 , DOI: 10.1039/d1dt03887g Kelly E Aldrich 1 , Ivan A Popov 1, 2 , Harrison D Root 1 , Enrique R Batista 1 , Samuel M Greer 1 , Stosh A Kozimor 1 , Laura M Lilley 1 , Maksim Y Livshits 1 , Veronika Mocko 1 , Michael T Janicke 1 , Brian L Scott 1 , Benjamin W Stein 1 , Ping Yang 1
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-06-24 , DOI: 10.1039/d1dt03887g Kelly E Aldrich 1 , Ivan A Popov 1, 2 , Harrison D Root 1 , Enrique R Batista 1 , Samuel M Greer 1 , Stosh A Kozimor 1 , Laura M Lilley 1 , Maksim Y Livshits 1 , Veronika Mocko 1 , Michael T Janicke 1 , Brian L Scott 1 , Benjamin W Stein 1 , Ping Yang 1
Affiliation
|
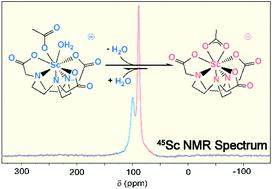
Developing chelators that strongly and selectively bind rare-earth elements (Sc, Y, La, and lanthanides) represents a longstanding fundamental challenge in inorganic chemistry. Solving these challenges is becoming more important because of increasing use of rare-earth elements in numerous technologies, ranging from paramagnets to luminescent materials. Within this context, we interrogated the complexation chemistry of the scandium(III) (Sc3+) trication with the hexadentate 1,4,7-triazacyclononane-1,4,7-triacetic acid (H3NOTA) chelator. This H3NOTA chelator is often regarded as an underperformer for complexing Sc3+. A common assumption is that metalation does not fully encapsulate Sc3+ within the NOTA3− macrocycle, leaving Sc3+ on the periphery of the chelate and susceptible to demetalation. Herein, we developed a synthetic approach that contradicted those assumptions. We confirmed that our procedure forced Sc3+ into the NOTA3− binding pocket by using single crystal X-ray diffraction to determine the Na[Sc(NOTA)(OOCCH3)] structure. Density functional theory (DFT) and 45Sc nuclear magnetic resonance (NMR) spectroscopy showed Sc3+ encapsulation was retained when the crystals were dissolved. Solution-phase and DFT studies revealed that [Sc(NOTA)(OOCCH3)]1− could accommodate an additional H2O capping ligand. Thermodynamic properties associated with the Sc-OOCCH3 and Sc-H2O capping ligand interactions demonstrated that these capping ligands occupied critical roles in stabilizing the [Sc(NOTA)] chelation complex.
中文翻译:

“笼内”钪-NOTA配合物的合成、固态、溶液和理论表征
开发能够强烈和选择性地结合稀土元素(Sc、Y、La 和镧系元素)的螯合剂是无机化学中长期存在的基本挑战。由于从顺磁体到发光材料等众多技术中越来越多地使用稀土元素,解决这些挑战变得越来越重要。在此背景下,我们研究了钪 ( III ) (Sc 3+ ) 三阳离子与六齿 1,4,7-三氮杂环壬烷-1,4,7-三乙酸 (H 3 NOTA) 螯合剂的络合化学。这种 H 3 NOTA 螯合剂通常被认为在络合 Sc 3+方面表现不佳。一个常见的假设是金属化不能完全封装 Sc 3+在 NOTA 3−大环内,Sc 3+留在螯合物的外围,容易脱金属。在这里,我们开发了一种与这些假设相矛盾的综合方法。我们证实,我们的程序通过使用单晶 X 射线衍射确定 Na[Sc(NOTA)(OOCCH 3 )] 结构,迫使 Sc 3+进入 NOTA 3-结合袋。密度泛函理论 (DFT) 和45 Sc 核磁共振 (NMR) 光谱表明,当晶体溶解时,Sc 3+封装得以保留。求解阶段和 DFT 研究表明 [Sc(NOTA)(OOCCH 3 )] 1−可以容纳额外的 H2 O 封端配体。与 Sc-OOCCH 3和 Sc-H 2 O 封端配体相互作用相关的热力学性质表明,这些封端配体在稳定 [Sc(NOTA)] 螯合复合物方面起着关键作用。
更新日期:2022-06-24
中文翻译:

“笼内”钪-NOTA配合物的合成、固态、溶液和理论表征
开发能够强烈和选择性地结合稀土元素(Sc、Y、La 和镧系元素)的螯合剂是无机化学中长期存在的基本挑战。由于从顺磁体到发光材料等众多技术中越来越多地使用稀土元素,解决这些挑战变得越来越重要。在此背景下,我们研究了钪 ( III ) (Sc 3+ ) 三阳离子与六齿 1,4,7-三氮杂环壬烷-1,4,7-三乙酸 (H 3 NOTA) 螯合剂的络合化学。这种 H 3 NOTA 螯合剂通常被认为在络合 Sc 3+方面表现不佳。一个常见的假设是金属化不能完全封装 Sc 3+在 NOTA 3−大环内,Sc 3+留在螯合物的外围,容易脱金属。在这里,我们开发了一种与这些假设相矛盾的综合方法。我们证实,我们的程序通过使用单晶 X 射线衍射确定 Na[Sc(NOTA)(OOCCH 3 )] 结构,迫使 Sc 3+进入 NOTA 3-结合袋。密度泛函理论 (DFT) 和45 Sc 核磁共振 (NMR) 光谱表明,当晶体溶解时,Sc 3+封装得以保留。求解阶段和 DFT 研究表明 [Sc(NOTA)(OOCCH 3 )] 1−可以容纳额外的 H2 O 封端配体。与 Sc-OOCCH 3和 Sc-H 2 O 封端配体相互作用相关的热力学性质表明,这些封端配体在稳定 [Sc(NOTA)] 螯合复合物方面起着关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号