当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of a Thermally and Hydrothermally Stable Copper-Based Catalyst via Alloying of Cu with Ni and Zn for Catalyzing Conversion of Furfural into Cyclopentanone
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-06-24 , DOI: 10.1021/acssuschemeng.2c01082 Yuewen Shao 1 , Kai Sun 1 , Mengjiao Fan 1 , Guoming Gao 1 , Junzhe Wang 1 , Lijun Zhang 1 , Shu Zhang 2 , Xun Hu 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-06-24 , DOI: 10.1021/acssuschemeng.2c01082 Yuewen Shao 1 , Kai Sun 1 , Mengjiao Fan 1 , Guoming Gao 1 , Junzhe Wang 1 , Lijun Zhang 1 , Shu Zhang 2 , Xun Hu 1
Affiliation
|
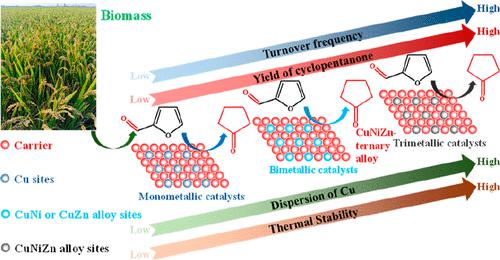
Copper-based catalysts are active for hydrogenation, while they are prone to sintering in hydrothermal conditions. In this study, alloying Cu with Ni and Zn was adopted to suppress sintering of copper species in the catalyzed conversion of furfural to cyclopentanone (CPO) and cyclopentanol (CPL) in the aqueous phase. The results indicated that Zn acted as a “glue” for aiding the formation of CuZn or CuNiZn alloy. The formation of the ternary CuNiZn alloy in CuNiZn/Al2O3 catalyst created the developed porous structure, induced the formation of abundant Lewis acidic sites and Brønsted acidic sites, and enhanced the dispersion and the total surface area of Cu species. These together rendered CuNiZn/Al2O3 with superior activity to monometallic or bimetallic Cu-based catalysts for the production of CPO and CPL (sum yield of 86% over Cu1.0Ni1.0Zn1.0/Al2O3), which was attributed to the synergy between CuNiZn alloy and Brønsted acidic sites. Alloying Ni with Cu and Zn weakened the adsorption of the C═C bond in the furan ring of furfural, while enhancing the activation of C═O and C–O–C bonds in furfural, avoiding the deep hydrogenation of the furan ring, facilitating the rearrangement of furfuryl alcohol to CPO, and improving the turnover frequency for the production of CPO from furfural remarkably (1011.3 h–1 over Cu1.0Ni1.0Zn1.0/Al2O3 vs 23.9 h–1 over Cu1.0/Al2O3). The formation of ternary CuNiZn alloy could not only suppress the sintering of metallic Cu species during the reduction in hydrogen but also effectively prevent the aggregation of Cu species in the aqueous phase, enhancing the catalytic stability under hydrothermal conditions.
中文翻译:

铜与镍、锌合金化合成热和水热稳定的铜基催化剂用于催化糠醛转化为环戊酮
铜基催化剂具有加氢活性,但在水热条件下易于烧结。在这项研究中,在糠醛催化转化为水相中的环戊酮 (CPO) 和环戊醇 (CPL) 过程中,采用 Cu 与 Ni 和 Zn 合金化来抑制铜物种的烧结。结果表明,Zn作为“胶水”帮助CuZn或CuNiZn合金的形成。CuNiZn/Al 2 O 3催化剂中三元CuNiZn合金的形成形成了发达的多孔结构,诱导了丰富的Lewis酸性位点和Brønsted酸性位点的形成,提高了Cu物种的分散度和总表面积。这些共同构成了 CuNiZn/Al 2 O 3与单金属或双金属 Cu 基催化剂相比,用于生产 CPO 和 CPL 的活性更高(总产率为 86%,高于 Cu 1.0 Ni 1.0 Zn 1.0 /Al 2 O 3),这归因于 CuNiZn 合金和布朗斯台德酸性网站。Ni与Cu、Zn合金化减弱了糠醛呋喃环中C=C键的吸附,同时增强了糠醛中C=O和C-O-C键的活化,避免了呋喃环的深度氢化,有利于糠醇重排为 CPO,并显着提高糠醛生产 CPO 的周转频率 (1011.3 h -1 over Cu 1.0 Ni 1.0 Zn1.0 /Al 2 O 3与 23.9 h –1在 Cu 1.0 /Al 2 O 3上)。三元CuNiZn合金的形成不仅可以抑制金属Cu物种在氢气还原过程中的烧结,还可以有效防止Cu物种在水相中的聚集,提高水热条件下的催化稳定性。
更新日期:2022-06-24
中文翻译:

铜与镍、锌合金化合成热和水热稳定的铜基催化剂用于催化糠醛转化为环戊酮
铜基催化剂具有加氢活性,但在水热条件下易于烧结。在这项研究中,在糠醛催化转化为水相中的环戊酮 (CPO) 和环戊醇 (CPL) 过程中,采用 Cu 与 Ni 和 Zn 合金化来抑制铜物种的烧结。结果表明,Zn作为“胶水”帮助CuZn或CuNiZn合金的形成。CuNiZn/Al 2 O 3催化剂中三元CuNiZn合金的形成形成了发达的多孔结构,诱导了丰富的Lewis酸性位点和Brønsted酸性位点的形成,提高了Cu物种的分散度和总表面积。这些共同构成了 CuNiZn/Al 2 O 3与单金属或双金属 Cu 基催化剂相比,用于生产 CPO 和 CPL 的活性更高(总产率为 86%,高于 Cu 1.0 Ni 1.0 Zn 1.0 /Al 2 O 3),这归因于 CuNiZn 合金和布朗斯台德酸性网站。Ni与Cu、Zn合金化减弱了糠醛呋喃环中C=C键的吸附,同时增强了糠醛中C=O和C-O-C键的活化,避免了呋喃环的深度氢化,有利于糠醇重排为 CPO,并显着提高糠醛生产 CPO 的周转频率 (1011.3 h -1 over Cu 1.0 Ni 1.0 Zn1.0 /Al 2 O 3与 23.9 h –1在 Cu 1.0 /Al 2 O 3上)。三元CuNiZn合金的形成不仅可以抑制金属Cu物种在氢气还原过程中的烧结,还可以有效防止Cu物种在水相中的聚集,提高水热条件下的催化稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号