当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-Free Approach for the Degradation of Bio- and CO2-Sourced Polycarbonates: A Step toward a Circular Plastic Economy
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-06-23 , DOI: 10.1021/acssuschemeng.2c01891 Fabiana Siragusa 1, 2 , Thomas Habets 1 , Raphael Méreau 3 , Gwilherm Evano 2 , Bruno Grignard 1 , Christophe Detrembleur 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-06-23 , DOI: 10.1021/acssuschemeng.2c01891 Fabiana Siragusa 1, 2 , Thomas Habets 1 , Raphael Méreau 3 , Gwilherm Evano 2 , Bruno Grignard 1 , Christophe Detrembleur 1
Affiliation
|
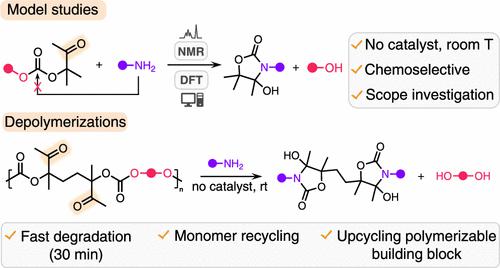
Designing easily degradable polymers has become a new challenge to overcome the post-consumer plastic waste accumulation in the environment. Polycarbonates are important polymers that can be chemically recycled; however, most often, their degradation requires high temperatures and/or the use of catalysts. In this work, we report the facile chemical recycling of regioregular polycarbonates prepared by the organocatalyzed copolymerization of CO2-sourced exovinylene biscyclic carbonates (BisαCC) with diols derived from biomass. These polymers, thanks to their pending ketone groups, are rapidly (<30 min) and totally deconstructed into the parent diol and a bis(oxazolidinone) by catalyst-free aminolysis at 25 °C. By using 3-propanolamine for the aminolysis, a hydroxy-functionalized bis(oxazolidinone) is recovered, which can be copolymerized with BisαCC to yield a polymer alternating carbonate and oxazolidinone linkages. Importantly, the same bis(oxazolidinone) scaffold is recovered as the main product by aminolysis of this copolymer, offering a close-loop recycling scenario for this polymer. This work illustrates that these polycarbonates are prone to facile and complete aminolysis under mild and catalyst-free conditions, but can also be exploited to prepare new building blocks for the synthesis of novel degradable polymers. The mechanism of formation of these heterocycles is studied by model reactions and rationalized by density functional theory (DFT) calculations.
中文翻译:

生物源和二氧化碳源聚碳酸酯的无催化剂降解方法:迈向循环塑料经济的一步
设计易于降解的聚合物已成为克服环境中消费后塑料废物积累的新挑战。聚碳酸酯是可以化学回收的重要聚合物;然而,大多数情况下,它们的降解需要高温和/或使用催化剂。在这项工作中,我们报道了通过 CO 2的有机催化共聚制备的区域规则聚碳酸酯的简便化学回收- 来源外亚乙烯基双环碳酸酯 (BisαCC) 与衍生自生物质的二醇。这些聚合物由于其悬置的酮基团,在 25 °C 下通过无催化剂氨解迅速(<30 分钟)并完全解构为母体二醇和双(恶唑烷酮)。通过使用3-丙醇胺进行氨解,回收羟基官能化的双(恶唑烷酮),它可以与BisαCC共聚以产生聚合物交替碳酸酯和恶唑烷酮键。重要的是,同样的双(恶唑烷酮)支架通过这种共聚物的氨解作为主要产品被回收,为这种聚合物提供了一个闭环回收方案。这项工作表明,这些聚碳酸酯在温和和无催化剂的条件下容易发生完全的氨解作用,但也可用于制备用于合成新型可降解聚合物的新结构单元。这些杂环的形成机制通过模型反应进行了研究,并通过密度泛函理论 (DFT) 计算进行了合理化。
更新日期:2022-06-23
中文翻译:

生物源和二氧化碳源聚碳酸酯的无催化剂降解方法:迈向循环塑料经济的一步
设计易于降解的聚合物已成为克服环境中消费后塑料废物积累的新挑战。聚碳酸酯是可以化学回收的重要聚合物;然而,大多数情况下,它们的降解需要高温和/或使用催化剂。在这项工作中,我们报道了通过 CO 2的有机催化共聚制备的区域规则聚碳酸酯的简便化学回收- 来源外亚乙烯基双环碳酸酯 (BisαCC) 与衍生自生物质的二醇。这些聚合物由于其悬置的酮基团,在 25 °C 下通过无催化剂氨解迅速(<30 分钟)并完全解构为母体二醇和双(恶唑烷酮)。通过使用3-丙醇胺进行氨解,回收羟基官能化的双(恶唑烷酮),它可以与BisαCC共聚以产生聚合物交替碳酸酯和恶唑烷酮键。重要的是,同样的双(恶唑烷酮)支架通过这种共聚物的氨解作为主要产品被回收,为这种聚合物提供了一个闭环回收方案。这项工作表明,这些聚碳酸酯在温和和无催化剂的条件下容易发生完全的氨解作用,但也可用于制备用于合成新型可降解聚合物的新结构单元。这些杂环的形成机制通过模型反应进行了研究,并通过密度泛函理论 (DFT) 计算进行了合理化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号