当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen Corrosion Engineering of Nonprecious Ternary Metal Hydroxides toward Oxygen Evolution Reaction
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-06-23 , DOI: 10.1021/acssuschemeng.2c02114 Yang Xiao 1 , Kamran Dastafkan 1 , Yibing Li 1 , Tingwen Zhao 1 , Zhen Su 1 , Huiqian Qi 2 , Chuan Zhao 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-06-23 , DOI: 10.1021/acssuschemeng.2c02114 Yang Xiao 1 , Kamran Dastafkan 1 , Yibing Li 1 , Tingwen Zhao 1 , Zhen Su 1 , Huiqian Qi 2 , Chuan Zhao 1
Affiliation
|
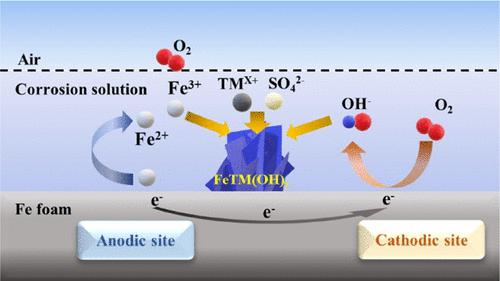
Facile electrocatalyst development with minimum energy consumption is highly beneficial for practical water splitting. Scale up of lab-scale active catalysts presents challenges to this end. Here, we take advantage of the spontaneous corrosion electrochemistry to make nonprecious multi-metallic hydroxides for efficient oxygen evolution reaction in alkaline electrolytes. Ternary FeNiCr and FeCoCr hydroxides are developed by oxygen- and sulfate-mediated corrosion engineering of macroporous Fe foam substrates. Cr doping is successfully achieved for regulating and accelerating the in situ phase transformation of Ni active sites into oxyhydroxide active phase to afford high intrinsic electrocatalytic activity and surface-intermediate interactions. Low overpotentials of 323 and 329 mV are achieved for delivering a large current density of 500 mA cm–2 using FeNiCr and FeCoCr electrocatalysts, respectively. Long-term stability at large current densities, reproducible performance, and facile scale up obtained suggests a great potential of designing highly efficient multi-metal electrocatalysts for water electrolysis technologies by corrosion engineering.
中文翻译:

非贵重三元金属氢氧化物析氧反应的氧腐蚀工程
以最小的能耗开发简便的电催化剂对于实际的水分解非常有益。实验室规模的活性催化剂的放大为此提出了挑战。在这里,我们利用自发腐蚀电化学制备非贵重多金属氢氧化物,以在碱性电解质中进行高效的析氧反应。三元 FeNiCr 和 FeCoCr 氢氧化物是通过氧和硫酸盐介导的大孔 Fe 泡沫基材腐蚀工程开发的。成功地实现了 Cr 掺杂,以调节和加速 Ni 活性位点原位相转变为羟基氧化物活性相,从而提供高本征电催化活性和表面-中间体相互作用。实现了 323 和 329 mV 的低过电势,可提供 500 mA cm 的大电流密度–2分别使用 FeNiCr 和 FeCoCr 电催化剂。在大电流密度下的长期稳定性、可重复的性能和易于放大表明,通过腐蚀工程设计用于水电解技术的高效多金属电催化剂具有巨大潜力。
更新日期:2022-06-23
中文翻译:

非贵重三元金属氢氧化物析氧反应的氧腐蚀工程
以最小的能耗开发简便的电催化剂对于实际的水分解非常有益。实验室规模的活性催化剂的放大为此提出了挑战。在这里,我们利用自发腐蚀电化学制备非贵重多金属氢氧化物,以在碱性电解质中进行高效的析氧反应。三元 FeNiCr 和 FeCoCr 氢氧化物是通过氧和硫酸盐介导的大孔 Fe 泡沫基材腐蚀工程开发的。成功地实现了 Cr 掺杂,以调节和加速 Ni 活性位点原位相转变为羟基氧化物活性相,从而提供高本征电催化活性和表面-中间体相互作用。实现了 323 和 329 mV 的低过电势,可提供 500 mA cm 的大电流密度–2分别使用 FeNiCr 和 FeCoCr 电催化剂。在大电流密度下的长期稳定性、可重复的性能和易于放大表明,通过腐蚀工程设计用于水电解技术的高效多金属电催化剂具有巨大潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号