Water Research ( IF 11.4 ) Pub Date : 2022-06-23 , DOI: 10.1016/j.watres.2022.118789 Mo Li 1 , Qinxue Wen 2 , Yongming Zhang 1 , Zhiqiang Chen 2
|
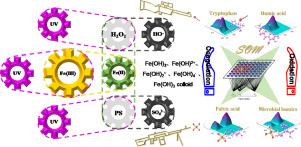
The negative effects of effluent organic matter (EfOM) on receiving aquatic environments and advanced treatment facilities pose significant concerns. However, the effective removal of EfOM is challenging due to its chemically complex nature and its refractory characteristics. In this study, two Fe(II)-assisted oxidation processes including UV/Fe(II)/H2O2 and UV/Fe(II)/persulfate (UV/Fe(II)/PS) were investigated to promote EfOM reduction. Fe(II) was essential for promoting EfOM degradation. The mineralization rate of EfOM increased from 7 to 29% with 2 mM Fe(II) addition in the UV/H2O2 process and to 23% with 0.8 mM Fe(II) addition in the UV/PS process. A preliminary experiment was conducted to obtain the optimal molar ratio of oxidant to Fe(II) for practical applications based on different indicators. The form of Fe(III) prevalent at different pH values strongly affected Fe(II)/Fe(III) cycling, thus determining the progress of EfOM degradation. A machine learning approach consisting of parallel factor analysis coupled with self-organizing maps (PARAFAC-SOM) was employed with fluorescence spectra to visualize the degradation behavior of EfOM in the different reaction systems. Four components (i.e., two humic-like substances, one fulvic acid, and one tryptophan-like substance) were eventually identified, and their reductions reached more than 62% during the Fe(II)-assisted oxidation processes. The degradation orders for each component in the different oxidation processes were initially evaluated by SOM analysis with Fmax percentage data. The degradation behavior of EfOM in the UV/Fe(II)/H2O2 and UV/Fe(II)/PS systems exhibited different trends based on the best matching unit map and component planes. The humic-like component was more refractory than the other three components in both oxidation processes. The microbial humic-like and high-molecular-weight fulvic acid substances showed higher reactivity with SO·4- than with ·OH, while the tryptophan-like substance was more reactive in the UV/Fe(II)/H2O2 system than in the UV/Fe(II)/PS system. The outcomes of this study provide new insights into the degradation behavior of EfOM, promoting the development of advanced wastewater treatments.
中文翻译:

Fe(II) 辅助高级氧化过程中流出物有机物转化的新见解:并行因子分析与自组织图相结合
流出物有机物 (EfOM) 对接收水环境和先进处理设施的负面影响引起了重大关注。然而,由于 EfOM 的化学复杂性和耐火特性,有效去除 EfOM 具有挑战性。在这项研究中,研究了两种 Fe(II) 辅助氧化过程,包括 UV/Fe(II)/H 2 O 2和 UV/Fe(II)/过硫酸盐 (UV/Fe(II)/PS),以促进 EfOM 还原. Fe(II) 对于促进 EfOM 降解至关重要。在 UV/H 2 O 2中添加 2 mM Fe(II),EfOM 的矿化率从 7% 增加到 29%在 UV/PS 工艺中添加 0.8 mM Fe(II) 时达到 23%。进行了初步实验,以根据不同的指标获得适合实际应用的氧化剂与 Fe(II) 的最佳摩尔比。在不同 pH 值下普遍存在的 Fe(III) 的形式强烈影响 Fe(II)/Fe(III) 循环,从而决定了 EfOM 降解的进程。由并行因子分析和自组织图 (PARAFAC-SOM) 组成的机器学习方法与荧光光谱一起使用,以可视化 EfOM 在不同反应系统中的降解行为。最终鉴定出四种成分(即两种腐殖质类物质、一种黄腐酸和一种类色氨酸物质),在 Fe(II) 辅助氧化过程中它们的还原率达到 62% 以上。F最大百分比数据。基于最佳匹配单元图和组件平面,EfOM 在 UV/Fe(II)/H 2 O 2和 UV/Fe(II)/PS 系统中的降解行为表现出不同的趋势。在两种氧化过程中,类腐殖质成分比其他三种成分更难降解。微生物类腐殖质和高分子量富里酸物质与SO· 4 -的反应性高于与·OH 的反应性,而类色氨酸物质在UV/Fe(II)/H 2 O 2中的反应性更强系统比在 UV/Fe(II)/PS 系统中。这项研究的结果为 EfOM 的降解行为提供了新的见解,促进了先进废水处理的发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号