当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
TEMPO-Promoted Mono- and Bisimidation of Tertiary Anilines: Synthesis of Symmetric and Unsymmetric N-Mannich Bases
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.joc.2c00700 Xiu Juan Xu 1 , Adila Amuti 1 , Wen Jing Hu 1 , Qiaerbati Adelibieke 1 , Abudureheman Wusiman 1, 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.joc.2c00700 Xiu Juan Xu 1 , Adila Amuti 1 , Wen Jing Hu 1 , Qiaerbati Adelibieke 1 , Abudureheman Wusiman 1, 2
Affiliation
|
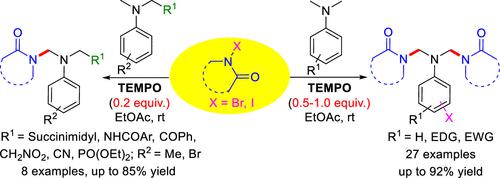
A TEMPO-promoted method was developed for the synthesis of symmetric bis-N-Mannich bases via sequential activation of two α,α′-amino C(sp3)–H bonds of N,N-dimethylanilines under mild conditions. This methodology was further extended for monoimidation of α-amino-functionalized methylanilines to give unsymmetric N-Mannich bases in good to high yields. Several control experiments were performed, and the coupling reaction outcomes indicated that the oxoammonium (TEMPO+) species is involved in the reaction.
中文翻译:

TEMPO 促进叔苯胺的单亚胺化和双亚胺化:对称和不对称 N-曼尼希碱基的合成
开发了一种 TEMPO 促进的方法,用于在温和条件下通过顺序激活N , N-二甲基苯胺的两个 α,α'-氨基 C(sp 3 )-H 键来合成对称双-N-曼尼希碱基。该方法进一步扩展用于 α-氨基官能化甲基苯胺的单酰亚胺化,以良好至高产率提供不对称N-曼尼希碱。进行了几个对照实验,偶联反应结果表明氧铵 (TEMPO + ) 物质参与了反应。
更新日期:2022-06-24
中文翻译:

TEMPO 促进叔苯胺的单亚胺化和双亚胺化:对称和不对称 N-曼尼希碱基的合成
开发了一种 TEMPO 促进的方法,用于在温和条件下通过顺序激活N , N-二甲基苯胺的两个 α,α'-氨基 C(sp 3 )-H 键来合成对称双-N-曼尼希碱基。该方法进一步扩展用于 α-氨基官能化甲基苯胺的单酰亚胺化,以良好至高产率提供不对称N-曼尼希碱。进行了几个对照实验,偶联反应结果表明氧铵 (TEMPO + ) 物质参与了反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号