当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioisomerism in Symmetric Dimethyl Dialdehydes Dictates their Photochemical Reactivity
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.joc.2c01020 Florian Feist 1 , Leona L Rodrigues 2, 3 , Sarah L Walden 2, 3 , Christopher Barner-Kowollik 1, 2, 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.joc.2c01020 Florian Feist 1 , Leona L Rodrigues 2, 3 , Sarah L Walden 2, 3 , Christopher Barner-Kowollik 1, 2, 3
Affiliation
|
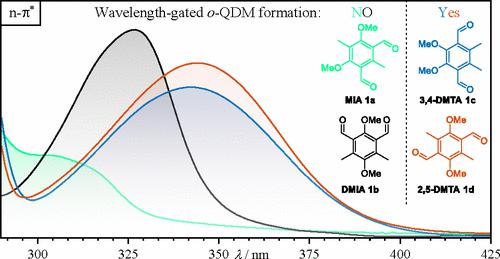
We herein report the first light-driven selective monoderivatization (desymmetrization) of two chemically equivalent carbonyl groups in a single chromophore. By comparing of four symmetric regioisomers, featuring two equivalent ortho-methylbenzaldehyde units, we identify dimethyltherephtalaldehydes (DMTAs) which can be activated in a dual wavelength-selective fashion. Under visible light and UV-light irradiation, DMTAs undergo two consecutive Diels–Alder reactions exhibiting near-quantitative endo-selectivity (>99%) and provide excellent yields (96–98%). The influence of the regioisomerism of the dialdehydes on their photochemical behavior is profound, evidenced by an in-depth investigation of their photochemical performance. We exemplify the capability of the photosystems via the synthesis of complex Diels–Alder adducts with various dienophiles, including alkynes.
中文翻译:

对称二甲基二醛中的区域异构决定了它们的光化学反应性
我们在此报告了单个生色团中两个化学等价羰基的第一次光驱动选择性单衍生化(去对称化)。通过比较具有两个等效邻甲基苯甲醛单元的四种对称区域异构体,我们确定了可以以双波长选择性方式激活的二甲基对苯二甲醛 (DMTA)。在可见光和紫外光照射下,DMTA 经历了两个连续的 Diels-Alder 反应,呈现出近定量的内切-选择性 (>99%) 并提供出色的收率 (96–98%)。二醛的区域异构对其光化学行为的影响是深远的,对其光化学性能的深入研究证明了这一点。我们通过与各种亲双烯体(包括炔烃)合成复杂的 Diels-Alder 加合物来举例说明光系统的能力。
更新日期:2022-06-24
中文翻译:

对称二甲基二醛中的区域异构决定了它们的光化学反应性
我们在此报告了单个生色团中两个化学等价羰基的第一次光驱动选择性单衍生化(去对称化)。通过比较具有两个等效邻甲基苯甲醛单元的四种对称区域异构体,我们确定了可以以双波长选择性方式激活的二甲基对苯二甲醛 (DMTA)。在可见光和紫外光照射下,DMTA 经历了两个连续的 Diels-Alder 反应,呈现出近定量的内切-选择性 (>99%) 并提供出色的收率 (96–98%)。二醛的区域异构对其光化学行为的影响是深远的,对其光化学性能的深入研究证明了这一点。我们通过与各种亲双烯体(包括炔烃)合成复杂的 Diels-Alder 加合物来举例说明光系统的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号