当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Unnatural α-Amino Acid Derivatives via Photoredox Activation of Inert C(sp3)–H Bonds
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.orglett.2c01822 Florence Babawale 1 , Kathiravan Murugesan 1 , Rok Narobe 1 , Burkhard König 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.orglett.2c01822 Florence Babawale 1 , Kathiravan Murugesan 1 , Rok Narobe 1 , Burkhard König 1
Affiliation
|
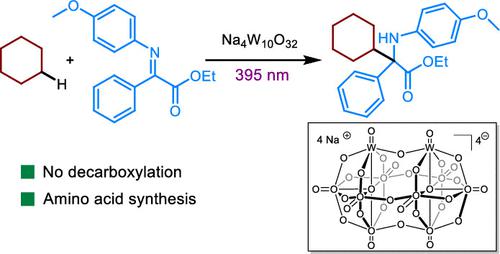
The synthesis of unnatural, tertiary amino acids is a challenging task. While decarboxylation–radical addition has been an important strategy for their formation, the use of alkyl radicals from C(sp3)–H bonds has not been fully explored. Herein, we report a photocatalytic protocol for the synthesis of unnatural α-amino esters employing abundant alkanes and imines retaining full atom economy. When this method is applied, several amino acid derivatives are synthesized in moderate to good yields.
中文翻译:

惰性 C(sp3)-H 键的光氧化还原活化合成非天然 α-氨基酸衍生物
非天然三级氨基酸的合成是一项具有挑战性的任务。虽然脱羧-自由基加成一直是它们形成的重要策略,但尚未充分探索使用来自 C(sp 3 )-H 键的烷基自由基。在此,我们报告了一种用于合成非天然 α-氨基酯的光催化方案,该方案使用丰富的烷烃和亚胺,保持全原子经济性。当应用这种方法时,可以以中等到高产率合成几种氨基酸衍生物。
更新日期:2022-06-24
中文翻译:

惰性 C(sp3)-H 键的光氧化还原活化合成非天然 α-氨基酸衍生物
非天然三级氨基酸的合成是一项具有挑战性的任务。虽然脱羧-自由基加成一直是它们形成的重要策略,但尚未充分探索使用来自 C(sp 3 )-H 键的烷基自由基。在此,我们报告了一种用于合成非天然 α-氨基酯的光催化方案,该方案使用丰富的烷烃和亚胺,保持全原子经济性。当应用这种方法时,可以以中等到高产率合成几种氨基酸衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号