Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring Interfacial Hydrolysis of Artificial Neutral Lipid Monolayer and Bilayer Catalyzed by Phospholipase C
Langmuir ( IF 3.7 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.langmuir.2c00995 Rongrong Du 1 , Xu Li 1 , Yong-Hao Ma 1 , Yongsheng Luo 1 , Chu Wang 1 , Qian Ma 2, 3 , Xiaolin Lu 1
Langmuir ( IF 3.7 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.langmuir.2c00995 Rongrong Du 1 , Xu Li 1 , Yong-Hao Ma 1 , Yongsheng Luo 1 , Chu Wang 1 , Qian Ma 2, 3 , Xiaolin Lu 1
Affiliation
|
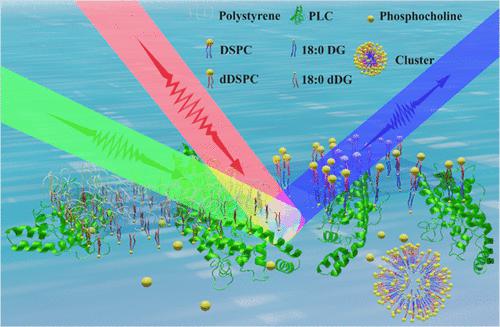
Phospholipase C (PLC) represents an important type of enzymes with the feature of hydrolyzing phospholipids at the position of the glycerophosphate bond, among which PLC extracted from Bacillus cereus (BC-PLC) has been extensively studied owing to its similarity to hitherto poorly characterized mammalian analogues. This study focuses on investigating the interfacial hydrolysis mechanism of phosphatidylcholine (PC) monolayer and bilayer membranes catalyzed by BC-PLC using sum frequency generation vibrational spectroscopy (SFG-VS) and laser scanning confocal microscopy (LSCM). We found that, upon interfacial hydrolysis, BC-PLC was adsorbed onto the lipid interface and catalyzed the lipolysis with no net orientation, as evidenced by the silent amide I band, indicating that ordered PLC alignment was not a prerequisite for the enzyme activity, which is very different from what we have reported for phospholipase A1 (PLA1) and phospholipase A2 (PLA2) [Kai, S. Phys. Chem. Chem. Phys. 2018, 20(1), 63−67; Wang, F. Langmuir 2019, 35(39), 12831–12838; Zhang, F. Langmuir 2020, 36(11), 2946–2953]. For the PC monolayer, one of the two hydrolysates, phosphocholine, desorbed from the interface into the aqueous phase, while the other one, diacylglycerol (DG), stayed well packed with high order at the interface. For the PC bilayer, phosphocholine dispersed into the aqueous phase too, similar to the monolayer case; however, DG, presumably formed clusters with the unreacted lipid substrates and desorbed from the interface. With respect to both the monolayer and bilayer cases, mechanistic schematics were presented to illustrate the different interfacial hydrolysis processes. Therefore, this model experimental study in vitro provides significant molecular-level insights and contributes necessary knowledge to reveal the lipolysis kinetics with respect to PLC and lipid membranes with monolayer and bilayer structures.
中文翻译:

探索磷脂酶C催化人工中性脂质单分子层和双层的界面水解
磷脂酶 C (PLC) 是一类重要的酶,具有水解甘油磷酸键位置的磷脂的特点,其中从蜡状芽孢杆菌中提取的 PLC(BC-PLC)由于其与迄今为止表征不佳的哺乳动物类似物的相似性而被广泛研究。本研究的重点是使用和频振动光谱 (SFG-VS) 和激光扫描共聚焦显微镜 (LSCM) 研究 BC-PLC 催化的磷脂酰胆碱 (PC) 单层和双层膜的界面水解机制。我们发现,在界面水解时,BC-PLC 被吸附到脂质界面上并催化脂解而没有净取向,正如沉默的酰胺 I 带所证明的那样,表明有序的 PLC 排列不是酶活性的先决条件,这与我们报道的磷脂酶 A 1 (PLA 1 ) 和磷脂酶 A 2 (PLA 2) [凯,S. 物理。化学。化学。物理。 2018 , 20 (1), 63−67;王,F. 朗缪尔 2019 , 35 (39), 12831–12838;张,F. 朗缪尔 2020 , 36 (11), 2946–2953]。对于 PC 单分子层,两种水解产物中的一种磷酸胆碱从界面解吸到水相中,而另一种水解物二酰基甘油 (DG) 在界面处保持良好的高阶堆积。对于 PC 双层,磷酸胆碱也分散到水相中,类似于单层情况;然而,DG 可能与未反应的脂质底物形成簇并从界面解吸。对于单层和双层情况,给出了机理示意图来说明不同的界面水解过程。因此,本模型体外实验研究提供了重要的分子水平见解并提供了必要的知识来揭示关于 PLC 和具有单层和双层结构的脂质膜的脂肪分解动力学。
更新日期:2022-06-24
中文翻译:

探索磷脂酶C催化人工中性脂质单分子层和双层的界面水解
磷脂酶 C (PLC) 是一类重要的酶,具有水解甘油磷酸键位置的磷脂的特点,其中从蜡状芽孢杆菌中提取的 PLC(BC-PLC)由于其与迄今为止表征不佳的哺乳动物类似物的相似性而被广泛研究。本研究的重点是使用和频振动光谱 (SFG-VS) 和激光扫描共聚焦显微镜 (LSCM) 研究 BC-PLC 催化的磷脂酰胆碱 (PC) 单层和双层膜的界面水解机制。我们发现,在界面水解时,BC-PLC 被吸附到脂质界面上并催化脂解而没有净取向,正如沉默的酰胺 I 带所证明的那样,表明有序的 PLC 排列不是酶活性的先决条件,这与我们报道的磷脂酶 A 1 (PLA 1 ) 和磷脂酶 A 2 (PLA 2) [











































 京公网安备 11010802027423号
京公网安备 11010802027423号