当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polyhedral Organic Silsesquioxane Cage Structures Formed via Reaction of Methylchlorosilanes with Re/CeO2 in Catalytic Oxygen Depletion–Regeneration Cycle
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.iecr.2c00920 Robert T. Larsen 1 , Matthew McLaughlin 1 , Dimitris E. Katsoulis 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-06-24 , DOI: 10.1021/acs.iecr.2c00920 Robert T. Larsen 1 , Matthew McLaughlin 1 , Dimitris E. Katsoulis 1
Affiliation
|
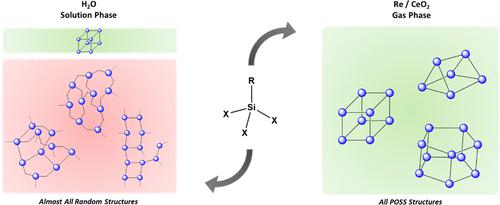
Polyhedral oligomeric silsesquioxane (POSS) cage structures were exclusively generated as the reaction products of methyltrichlorosilane, CH3SiCl3 (or MeSiCl3 where Me denotes a CH3-group), with a supported Re/CeO2 catalyst at 500 °C. The cage structures are generated by the exchange of oxygen for chlorine in the ceria support under a H2 reducing atmosphere. Regeneration of the support can occur by passing air over the catalyst at 500 °C, and then the reaction can be repeated. This presents a novel way of generating cage structures exclusively via a gas-phase reaction. Gas chromatography-mass spectrometry (GC-MS) of the reaction products indicates that the cage structures that are formed are Me6-T6, Me8-T8, and Me10-T10. The reaction proceeds at high conversion and high yield toward these POSS cages. When dimethyldichlorosilane ((CH3)2SiCl2 or Me2SiCl2) is used as the reactant, mostly cyclic siloxanes D4–D6 are produced. Hydrido-chlorosilane, methyldichlorosilane (CH3SiHCl2 or MeHSiCl2), generates cage structures through an additional Si–H–O ligand exchange reaction that is facilitated by the oxygen vacancies on the ceria support. When Si–H ligands are present, rearrangement reactions are also observed under these conditions and constitute a supplementary process for the formation of the POSS cage structures. Trichlorosilane (HSiCl3) does not form cage structures, but it does undergo rearrangement. It also undergoes Si–H–O ligand exchange with oxygen vacancies on the ceria support to form cristobalite (crystalline SiO2) on the support.
中文翻译:

甲基氯硅烷与 Re/CeO2 在催化耗氧-再生循环中反应形成的多面体有机倍半硅氧烷笼状结构
多面体低聚倍半硅氧烷 (POSS) 笼状结构仅作为甲基三氯硅烷 CH 3 SiCl 3(或 MeSiCl 3,其中 Me 表示 CH 3 -基团)与负载型 Re/CeO 2催化剂在 500°C 的反应产物产生。笼状结构是通过在 H 2下氧化铈载体中的氯与氧的交换而产生的还原气氛。载体的再生可以通过在 500 °C 下将空气通过催化剂进行,然后可以重复反应。这提出了一种专门通过气相反应生成笼状结构的新方法。反应产物的气相色谱-质谱(GC-MS)表明形成的笼状结构是Me 6 -T 6、Me 8 -T 8和Me 10 -T 10。反应以高转化率和高产率向这些 POSS 笼进行。当二甲基二氯硅烷 ((CH 3 ) 2 SiCl 2或 Me 2 SiCl 2) 用作反应物,主要生成环状硅氧烷 D 4 -D 6。氢化-氯硅烷,甲基二氯硅烷(CH 3 SiHCl 2或 MeHSiCl 2)通过额外的 Si-H-O 配体交换反应生成笼状结构,该反应由二氧化铈载体上的氧空位促进。当存在 Si-H 配体时,在这些条件下也会观察到重排反应,并构成形成 POSS 笼结构的补充过程。三氯硅烷 (HSiCl 3 ) 不会形成笼状结构,但会发生重排。它还与氧化铈载体上的氧空位进行 Si-H-O 配体交换,形成方英石(结晶 SiO2)关于支持。
更新日期:2022-06-24
中文翻译:

甲基氯硅烷与 Re/CeO2 在催化耗氧-再生循环中反应形成的多面体有机倍半硅氧烷笼状结构
多面体低聚倍半硅氧烷 (POSS) 笼状结构仅作为甲基三氯硅烷 CH 3 SiCl 3(或 MeSiCl 3,其中 Me 表示 CH 3 -基团)与负载型 Re/CeO 2催化剂在 500°C 的反应产物产生。笼状结构是通过在 H 2下氧化铈载体中的氯与氧的交换而产生的还原气氛。载体的再生可以通过在 500 °C 下将空气通过催化剂进行,然后可以重复反应。这提出了一种专门通过气相反应生成笼状结构的新方法。反应产物的气相色谱-质谱(GC-MS)表明形成的笼状结构是Me 6 -T 6、Me 8 -T 8和Me 10 -T 10。反应以高转化率和高产率向这些 POSS 笼进行。当二甲基二氯硅烷 ((CH 3 ) 2 SiCl 2或 Me 2 SiCl 2) 用作反应物,主要生成环状硅氧烷 D 4 -D 6。氢化-氯硅烷,甲基二氯硅烷(CH 3 SiHCl 2或 MeHSiCl 2)通过额外的 Si-H-O 配体交换反应生成笼状结构,该反应由二氧化铈载体上的氧空位促进。当存在 Si-H 配体时,在这些条件下也会观察到重排反应,并构成形成 POSS 笼结构的补充过程。三氯硅烷 (HSiCl 3 ) 不会形成笼状结构,但会发生重排。它还与氧化铈载体上的氧空位进行 Si-H-O 配体交换,形成方英石(结晶 SiO2)关于支持。










































 京公网安备 11010802027423号
京公网安备 11010802027423号