当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and structural characterization of copper–cuprizone complexes
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-06-23 , DOI: 10.1039/d2dt01475k M Jake Pushie 1 , Kelly L Summers 2 , Kurt H Nienaber 3 , Ingrid J Pickering 4, 5 , Graham N George 4, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-06-23 , DOI: 10.1039/d2dt01475k M Jake Pushie 1 , Kelly L Summers 2 , Kurt H Nienaber 3 , Ingrid J Pickering 4, 5 , Graham N George 4, 5
Affiliation
|
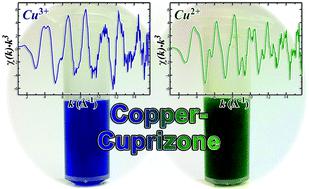
Copper(II) coordination by bis(cyclohexanone)oxalyldihydrazone (also known as cuprizone), resulting in the formation of an intensely coloured blue complex, was first reported over 70 years ago. The cuprizone reaction has been employed in colourimetric tests for the presence of trace levels of copper. Cuprizone administration in C57BL/6 mice also leads to demyelination over time – a consequence that appears to be due to copper dyshomeostasis – and this has led to use of cuprizone as the leading method for toxicant-induced generation of an animal model of demyelination since its first use in the 1960s. Despite broad interest in cuprizone and its ability to bind copper there have been relatively few studies to structurally characterize the copper coordination properties of this ligand. In the absence of an aqueous medium, such as neat alcohol, copper and cuprizone exclusively form an amorphous green precipitate. Under aqueous conditions, where a large excess of cuprizone (relative to copper) is present, the blue complex that is synonymous with copper–cuprizone coordination is predominantly formed. The blue and green copper–cuprizone products demonstrate poor solubility and present challenges for conventional structure characterization methods, such as X-ray crystallography or nuclear magnetic resonance spectroscopy. By combining mass spectrometry, X-ray absorption spectroscopy, computational chemistry, and other techniques, a self-consistent picture of the copper coordination structures of the blue and green complexes is revealed – confirming that the blue complex is in the Cu(III) state, containing two hydrolyzed cuprizone ligands per metal centre, while the green complex represents an extended oligomeric complex, comprised of repeating Cu(II) centres that lie 4.8 Å apart and are bridged by unhydrolyzed cuprizone donors.
中文翻译:

铜-铜酮配合物的合成与结构表征
铜(Ⅱ) 通过双(环己酮)草酰二腙(也称为铜酮)的配位,导致形成强烈的蓝色配合物,最早在 70 多年前被报道。铜酮反应已用于比色测试中是否存在痕量铜。在 C57BL/6 小鼠中施用铜宗酮也会随着时间的推移导致脱髓鞘——这一结果似乎是由于铜的体内平衡失调造成的——这导致铜宗用作毒物诱导的脱髓鞘动物模型生成的主要方法,因为它1960年代首次使用。尽管对铜酮及其结合铜的能力有广泛的兴趣,但在结构上表征该配体的铜配位特性的研究相对较少。在没有水性介质(例如纯酒精)的情况下,铜和铜宗仅形成无定形绿色沉淀。在含水条件下,存在大量过量的铜酮(相对于铜),主要形成与铜-铜酮配位同义的蓝色络合物。蓝色和绿色铜铜酮产物的溶解度较差,对传统的结构表征方法(如 X 射线晶体学或核磁共振光谱)提出了挑战。通过结合质谱、X 射线吸收光谱、计算化学和其他技术,揭示了蓝色和绿色配合物的铜配位结构的自洽图——证实蓝色配合物在 Cu( 如果存在大量过量的铜宗(相对于铜),则主要形成与铜-铜宗配位同义的蓝色络合物。蓝色和绿色铜铜酮产物的溶解度较差,对传统的结构表征方法(如 X 射线晶体学或核磁共振光谱)提出了挑战。通过结合质谱、X 射线吸收光谱、计算化学和其他技术,揭示了蓝色和绿色配合物的铜配位结构的自洽图——证实蓝色配合物在 Cu( 如果存在大量过量的铜宗(相对于铜),则主要形成与铜-铜宗配位同义的蓝色络合物。蓝色和绿色铜铜酮产物的溶解度较差,对传统的结构表征方法(如 X 射线晶体学或核磁共振光谱)提出了挑战。通过结合质谱、X 射线吸收光谱、计算化学和其他技术,揭示了蓝色和绿色配合物的铜配位结构的自洽图——证实蓝色配合物在 Cu( 蓝色和绿色铜铜酮产物的溶解度较差,对传统的结构表征方法(如 X 射线晶体学或核磁共振光谱)提出了挑战。通过结合质谱、X 射线吸收光谱、计算化学和其他技术,揭示了蓝色和绿色配合物的铜配位结构的自洽图——证实蓝色配合物在 Cu( 蓝色和绿色铜铜酮产物的溶解度较差,对传统的结构表征方法(如 X 射线晶体学或核磁共振光谱)提出了挑战。通过结合质谱、X 射线吸收光谱、计算化学和其他技术,揭示了蓝色和绿色配合物的铜配位结构的自洽图——证实蓝色配合物在 Cu(III ) 状态,每个金属中心包含两个水解的铜酮配体,而绿色复合物代表扩展的低聚复合物,由相距 4.8 Å的重复 Cu( II ) 中心组成,并由未水解的铜酮供体桥接。
更新日期:2022-06-23
中文翻译:

铜-铜酮配合物的合成与结构表征
铜(Ⅱ) 通过双(环己酮)草酰二腙(也称为铜酮)的配位,导致形成强烈的蓝色配合物,最早在 70 多年前被报道。铜酮反应已用于比色测试中是否存在痕量铜。在 C57BL/6 小鼠中施用铜宗酮也会随着时间的推移导致脱髓鞘——这一结果似乎是由于铜的体内平衡失调造成的——这导致铜宗用作毒物诱导的脱髓鞘动物模型生成的主要方法,因为它1960年代首次使用。尽管对铜酮及其结合铜的能力有广泛的兴趣,但在结构上表征该配体的铜配位特性的研究相对较少。在没有水性介质(例如纯酒精)的情况下,铜和铜宗仅形成无定形绿色沉淀。在含水条件下,存在大量过量的铜酮(相对于铜),主要形成与铜-铜酮配位同义的蓝色络合物。蓝色和绿色铜铜酮产物的溶解度较差,对传统的结构表征方法(如 X 射线晶体学或核磁共振光谱)提出了挑战。通过结合质谱、X 射线吸收光谱、计算化学和其他技术,揭示了蓝色和绿色配合物的铜配位结构的自洽图——证实蓝色配合物在 Cu( 如果存在大量过量的铜宗(相对于铜),则主要形成与铜-铜宗配位同义的蓝色络合物。蓝色和绿色铜铜酮产物的溶解度较差,对传统的结构表征方法(如 X 射线晶体学或核磁共振光谱)提出了挑战。通过结合质谱、X 射线吸收光谱、计算化学和其他技术,揭示了蓝色和绿色配合物的铜配位结构的自洽图——证实蓝色配合物在 Cu( 如果存在大量过量的铜宗(相对于铜),则主要形成与铜-铜宗配位同义的蓝色络合物。蓝色和绿色铜铜酮产物的溶解度较差,对传统的结构表征方法(如 X 射线晶体学或核磁共振光谱)提出了挑战。通过结合质谱、X 射线吸收光谱、计算化学和其他技术,揭示了蓝色和绿色配合物的铜配位结构的自洽图——证实蓝色配合物在 Cu( 蓝色和绿色铜铜酮产物的溶解度较差,对传统的结构表征方法(如 X 射线晶体学或核磁共振光谱)提出了挑战。通过结合质谱、X 射线吸收光谱、计算化学和其他技术,揭示了蓝色和绿色配合物的铜配位结构的自洽图——证实蓝色配合物在 Cu( 蓝色和绿色铜铜酮产物的溶解度较差,对传统的结构表征方法(如 X 射线晶体学或核磁共振光谱)提出了挑战。通过结合质谱、X 射线吸收光谱、计算化学和其他技术,揭示了蓝色和绿色配合物的铜配位结构的自洽图——证实蓝色配合物在 Cu(III ) 状态,每个金属中心包含两个水解的铜酮配体,而绿色复合物代表扩展的低聚复合物,由相距 4.8 Å的重复 Cu( II ) 中心组成,并由未水解的铜酮供体桥接。











































 京公网安备 11010802027423号
京公网安备 11010802027423号