当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deracemization of Binaphthyl by Suzuki Diarylation: The Role of Electronic and Steric Effects
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.joc.2c01041 Filip Bulko 1 , Michal Májek 1 , Martin Putala 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.joc.2c01041 Filip Bulko 1 , Michal Májek 1 , Martin Putala 1
Affiliation
|
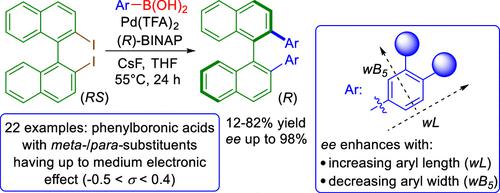
We report a Suzuki 2,2′-diarylation of the racemic 2,2′-diiodo-1,1′-binaphthyl which proceeds with deracemization via a pallada(IV)cyclic intermediate, induced by a simple chiral ligand─BINAP [2,2′-bis(diphenylphosphino)-1,1′,-binaphthyl]. A systematic study of the reaction scope, using 45 arylboronic acids, reveals that the diarylated product is formed when meta- and/or para-substituted phenylboronic acids are functionalized with a substituent with the Hammett constant from −0.5 to +0.4. Multiparametric analysis accounting for the effect of geometry on the reactivity using Boltzmann-weighted Sterimol parameters and electronic effects described by Hammett descriptors shows that the enantioselectivity depends on steric effects only, with enhanced enantioselectivity observed for substituents with a larger length, wL, and reduced for substituents with a larger maximum width, wB5. We show that careful tuning of these parameters, with the aid of the presented mathematical model, can lead to excellent enantioselectivity. Additional factors that are investigated and found to affect the stereoselective course of the reaction include the reaction temperature, palladium source, palladium to ligand ratio, and the type of boronic acid derivative. During the chromatographic separation of diarylated products on an achiral silica gel, we observed a rare phenomenon: the diarylated products undergo self-disproportionation of enantiomers, with the major enantiomer being eluted first.
中文翻译:

Suzuki Diarylation 对联萘的去外消旋:电子和空间效应的作用
我们报告了外消旋 2,2'-diiodo-1,1'-binaphthyl 的 Suzuki 2,2'-二芳基化反应,通过一个简单的手性配体─BINAP [2, 2'-双(二苯基膦基)-1,1',-联萘]。使用 45 种芳基硼酸对反应范围的系统研究表明,二芳基化产物是在间位和/或对位时形成的-取代的苯基硼酸被具有从-0.5到+0.4的哈米特常数的取代基官能化。使用玻尔兹曼加权 Sterimol 参数和 Hammett 描述符描述的电子效应对几何形状对反应性影响的多参数分析表明,对映选择性仅取决于空间效应,对于具有较大长度wL的取代基观察到增强的对映选择性,并减少最大宽度wB 5较大的取代基. 我们表明,借助所提出的数学模型,仔细调整这些参数可以产生出色的对映选择性。研究并发现影响反应立体选择性过程的其他因素包括反应温度、钯源、钯与配体的比例以及硼酸衍生物的类型。在非手性硅胶上二芳基化产物的色谱分离过程中,我们观察到一个罕见的现象:二芳基化产物发生对映异构体的自歧化,主要对映异构体首先被洗脱。
更新日期:2022-06-23
中文翻译:

Suzuki Diarylation 对联萘的去外消旋:电子和空间效应的作用
我们报告了外消旋 2,2'-diiodo-1,1'-binaphthyl 的 Suzuki 2,2'-二芳基化反应,通过一个简单的手性配体─BINAP [2, 2'-双(二苯基膦基)-1,1',-联萘]。使用 45 种芳基硼酸对反应范围的系统研究表明,二芳基化产物是在间位和/或对位时形成的-取代的苯基硼酸被具有从-0.5到+0.4的哈米特常数的取代基官能化。使用玻尔兹曼加权 Sterimol 参数和 Hammett 描述符描述的电子效应对几何形状对反应性影响的多参数分析表明,对映选择性仅取决于空间效应,对于具有较大长度wL的取代基观察到增强的对映选择性,并减少最大宽度wB 5较大的取代基. 我们表明,借助所提出的数学模型,仔细调整这些参数可以产生出色的对映选择性。研究并发现影响反应立体选择性过程的其他因素包括反应温度、钯源、钯与配体的比例以及硼酸衍生物的类型。在非手性硅胶上二芳基化产物的色谱分离过程中,我们观察到一个罕见的现象:二芳基化产物发生对映异构体的自歧化,主要对映异构体首先被洗脱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号