当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
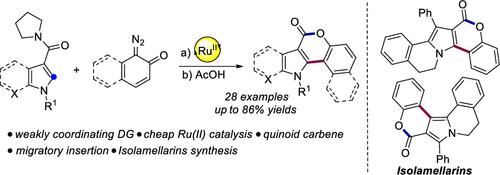
Weakly Coordinating tert-Amide-Assisted Ru(II)-Catalyzed Synthesis of Azacoumestans via Migratory Insertion of Quinoid Carbene: Application in the Total Synthesis of Isolamellarins
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.orglett.2c01556 Souradip Sarkar 1 , Rajarshi Samanta 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.orglett.2c01556 Souradip Sarkar 1 , Rajarshi Samanta 1
Affiliation
|
A weakly coordinating tert-amide-directed straightforward method was developed for the synthesis of azacoumestans using the corresponding azaheterocycle derivatives and diazonaphthoquinones under cheap Ru(II)-catalyzed conditions. The reaction proceeds via migratory insertion of quinoid carbene and subsequent Brønstead acid-mediated cyclization. The optimized C2-selective method offered a wide scope of important azaheterocycles. Bioactive natural products like isolamellarins A and B were synthesized via the developed protocol. Preliminary mechanistic studies highlighted the probable mechanistic pathway.
中文翻译:

弱配位叔酰胺辅助钌(II)-通过迁移插入醌卡宾催化合成氮杂香豆素:在异酚醛全合成中的应用
开发了一种弱配位叔酰胺定向的直接方法,用于在廉价的 Ru(II) 催化条件下使用相应的氮杂杂环衍生物和重氮萘醌合成氮杂香豆素。该反应通过醌型卡宾的迁移插入和随后的布朗斯特酸介导的环化进行。优化的 C2 选择性方法提供了广泛的重要氮杂杂环化合物。通过开发的方案合成了生物活性天然产物,如异醇 A 和 B。初步机制研究强调了可能的机制途径。
更新日期:2022-06-23
中文翻译:

弱配位叔酰胺辅助钌(II)-通过迁移插入醌卡宾催化合成氮杂香豆素:在异酚醛全合成中的应用
开发了一种弱配位叔酰胺定向的直接方法,用于在廉价的 Ru(II) 催化条件下使用相应的氮杂杂环衍生物和重氮萘醌合成氮杂香豆素。该反应通过醌型卡宾的迁移插入和随后的布朗斯特酸介导的环化进行。优化的 C2 选择性方法提供了广泛的重要氮杂杂环化合物。通过开发的方案合成了生物活性天然产物,如异醇 A 和 B。初步机制研究强调了可能的机制途径。









































 京公网安备 11010802027423号
京公网安备 11010802027423号