当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiocyanatoarylation of Methyl Vinyl Ketone under Meerwein Conditions for the Synthesis of 2-Aminothiazole-Based Heterocyclic Systems
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.orglett.2c01677 Yurii V Ostapiuk 1 , Oksana V Barabash 1 , Mary Y Ostapiuk 1 , Evgeny Goreshnik 2 , Mykola D Obushak 1 , Andreas Schmidt 3
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.orglett.2c01677 Yurii V Ostapiuk 1 , Oksana V Barabash 1 , Mary Y Ostapiuk 1 , Evgeny Goreshnik 2 , Mykola D Obushak 1 , Andreas Schmidt 3
Affiliation
|
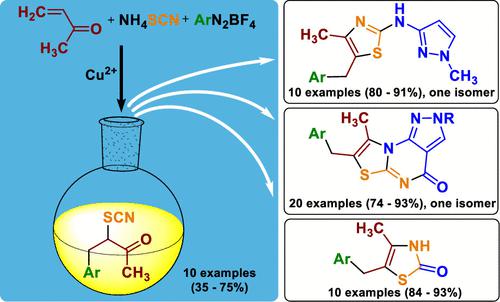
4-Aryl-3-thiocyanatobutan-2-ones were prepared by Meerwein reactions from methyl vinyl ketone and aryldiazonium salts under copper(II) catalysis in 35–75% yields. α-Thiocyanato ketones regioselectively react with 1-methyl-3-aminopyrazole forming N-(3-pyrazolyl)-substituted 2-aminothiazoles in 80–91% yields. An ester group in position 3 of the pyrazole induced a regioselective ring-closure reaction followed by an intramolecular cyclization, which gave first representatives of a new heterocyclic system, pyrazolo[4,3-e]thiazolo[3,2-a]pyrimidine, in 74–93% yields. In addition, the preparations of 5-benzyl-4-methylthiazol-2-ones in 84–93% yields are described.
中文翻译:

Meerwein 条件下甲基乙烯基酮的硫氰酸芳基化合成 2-氨基噻唑基杂环体系
4-Aryl-3-thiocyanatobutan-2-ones 是由甲基乙烯基酮和芳基重氮盐在铜 (II) 催化下的 Meerwein 反应制备的,收率 35-75%。α-硫氰酸根酮与 1-甲基-3-氨基吡唑发生区域选择性反应,形成N- (3-吡唑基)-取代的 2-氨基噻唑,产率为 80-91%。吡唑的 3 位上的酯基诱导区域选择性闭环反应,随后发生分子内环化,这产生了新杂环系统吡唑并[4,3 -e ]噻唑并[3,2- a ]嘧啶的第一个代表,产率为 74–93%。此外,还描述了 5-benzyl-4-methylthiazol-2-ones 的制备方法,产率为 84-93%。
更新日期:2022-06-23
中文翻译:

Meerwein 条件下甲基乙烯基酮的硫氰酸芳基化合成 2-氨基噻唑基杂环体系
4-Aryl-3-thiocyanatobutan-2-ones 是由甲基乙烯基酮和芳基重氮盐在铜 (II) 催化下的 Meerwein 反应制备的,收率 35-75%。α-硫氰酸根酮与 1-甲基-3-氨基吡唑发生区域选择性反应,形成N- (3-吡唑基)-取代的 2-氨基噻唑,产率为 80-91%。吡唑的 3 位上的酯基诱导区域选择性闭环反应,随后发生分子内环化,这产生了新杂环系统吡唑并[4,3 -e ]噻唑并[3,2- a ]嘧啶的第一个代表,产率为 74–93%。此外,还描述了 5-benzyl-4-methylthiazol-2-ones 的制备方法,产率为 84-93%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号