当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ketone–Olefin Coupling of Aliphatic and Aromatic Carbonyls Catalyzed by Excited-State Acridine Radicals
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-06-23 , DOI: 10.1021/jacs.2c04822 Nicholas J Venditto 1 , Yiyang S Liang 1 , Roukaya K El Mokadem 1 , David A Nicewicz 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-06-23 , DOI: 10.1021/jacs.2c04822 Nicholas J Venditto 1 , Yiyang S Liang 1 , Roukaya K El Mokadem 1 , David A Nicewicz 1
Affiliation
|
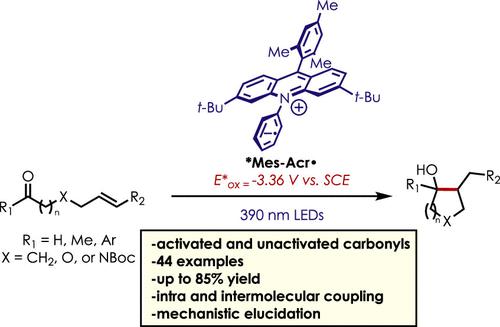
Ketone–olefin coupling reactions are common methods for the formation of carbon–carbon bonds. This reaction class typically requires stoichiometric or super stoichiometric quantities of metal reductants, and catalytic variations are limited in application. Photoredox catalysis has offered an alternative method toward ketone–olefin coupling reactions, although most methods are limited in scope to easily reducible aromatic carbonyl compounds. Herein, we describe a mild, metal-free ketone–olefin coupling reaction using an excited-state acridine radical super reductant as a photoredox catalyst. We demonstrate both intramolecular and intermolecular ketone–olefin couplings of aliphatic and aromatic ketones and aldehydes. Mechanistic evidence is also presented supporting an “olefin first” ketone–olefin coupling mechanism.
中文翻译:

激发态吖啶自由基催化脂肪族和芳香族羰基的酮-烯烃偶联
酮-烯烃偶联反应是形成碳-碳键的常用方法。该反应类别通常需要化学计量或超化学计量的金属还原剂,并且催化变化在应用中受到限制。光氧化还原催化为酮-烯烃偶联反应提供了一种替代方法,尽管大多数方法仅限于易于还原的芳香族羰基化合物。在这里,我们描述了一种温和的、无金属的酮-烯烃偶联反应,使用激发态吖啶自由基超级还原剂作为光氧化还原催化剂。我们展示了脂肪族和芳香族酮和醛的分子内和分子间酮-烯烃偶联。还提供了支持“烯烃优先”的机制证据酮-烯烃偶联机理。
更新日期:2022-06-23
中文翻译:

激发态吖啶自由基催化脂肪族和芳香族羰基的酮-烯烃偶联
酮-烯烃偶联反应是形成碳-碳键的常用方法。该反应类别通常需要化学计量或超化学计量的金属还原剂,并且催化变化在应用中受到限制。光氧化还原催化为酮-烯烃偶联反应提供了一种替代方法,尽管大多数方法仅限于易于还原的芳香族羰基化合物。在这里,我们描述了一种温和的、无金属的酮-烯烃偶联反应,使用激发态吖啶自由基超级还原剂作为光氧化还原催化剂。我们展示了脂肪族和芳香族酮和醛的分子内和分子间酮-烯烃偶联。还提供了支持“烯烃优先”的机制证据酮-烯烃偶联机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号