当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of Iridium(III) and Rhodium(III) Amine, Imine, and Amido Complexes Based on Pyridine–Amine Ligands: Structural Diversity Arising from Reaction Conditions, Substituent Variation, and Metal Centers
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.inorgchem.2c00984 Xueyan Hu 1 , Lihua Guo 1 , Mengqi Liu 1 , Mengru Sun 1 , Qiuya Zhang 1 , Hongwei Peng 1 , Fanjun Zhang 1 , Zhe Liu 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.inorgchem.2c00984 Xueyan Hu 1 , Lihua Guo 1 , Mengqi Liu 1 , Mengru Sun 1 , Qiuya Zhang 1 , Hongwei Peng 1 , Fanjun Zhang 1 , Zhe Liu 1
Affiliation
|
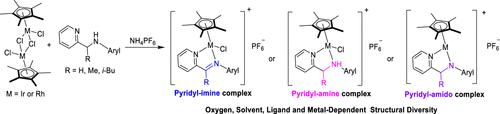
Herein, we present the different coordination modes of half-sandwich iridium(III) and rhodium(III) complexes based on pyridine–amine ligands. The pyridyl–amine iridium(III) and rhodium(III) complexes, the corresponding oxidation pyridyl–imine products, and 16-electron pyridyl–amido complexes can be obtained through the change in reaction conditions (nitrogen/adventitious oxygen atmosphere, reaction time, and solvents) and structural variations in the metal and ligand. Overall, the reaction of pyridine–amine ligands with [(η5-C5(CH3)5)MCl2]2 (M = Ir or Rh) in the presence of adventitious oxygen afforded the oxidized pyridyl–imine complexes. The possible mechanism for the oxidation of iridium(III) and rhodium(III) amine complexes was confirmed by the detection of the byproduct hydrogen peroxide. Moreover, the formation of pyridyl–amine complexes was favored when nonpolar solvent CH2Cl2 was used instead of CH3OH. The rarely reported complex with [(η5-Cp*)IrCl3] anions can also be obtained without the addition of NH4PF6. The introduction of the sterically bulky i-Bu group on the bridge carbon of the ligand led to the formation of stable 16-electron pyridyl–amido complexes. The pyridyl–amine iridium(III) and rhodium(III) complexes were also synthesized under a N2 atmosphere, and no H2O2 was detected in the whole process. In particular, the aqueous solution stability and in vitro cytotoxicity toward A549 and HeLa human cancer cells of these complexes were also evaluated. No obvious selectivity was observed for cancer cells versus normal cells with these complexes. Notably, the represented complex 5a can promote an increase in the reactive oxygen species level and induce cell death via apoptosis.
中文翻译:

基于吡啶-胺配体的铱 (III) 和铑 (III) 胺、亚胺和酰胺配合物的形成:由反应条件、取代基变化和金属中心引起的结构多样性
在此,我们展示了基于吡啶-胺配体的半夹心铱(III)和铑(III)配合物的不同配位模式。通过改变反应条件(氮气/外加氧气氛、反应时间、和溶剂)以及金属和配体的结构变化。总的来说,吡啶-胺配体与[(η 5 -C 5 (CH 3 ) 5 )MCl 2 ] 2的反应(M = Ir 或 Rh)在外来氧存在下提供氧化的吡啶基 - 亚胺复合物。铱(III) 和铑(III) 胺配合物的氧化可能机理通过副产物过氧化氢的检测得到证实。此外,当使用非极性溶剂 CH 2 Cl 2代替 CH 3 OH时,有利于形成吡啶基-胺络合物。很少报道的具有[(η 5 -Cp*)IrCl 3 ] 阴离子的络合物也可以在不添加NH 4 PF 6的情况下获得。空间大体积i的引入配体桥碳上的 -Bu 基团导致形成稳定的 16 电子吡啶基-酰胺络合物。吡啶基-胺铱(III)和铑(III)配合物也是在N 2气氛下合成的,整个过程中没有检测到H 2 O 2 。特别是,还评估了这些复合物对 A549 和 HeLa 人癌细胞的水溶液稳定性和体外细胞毒性。对于癌细胞与具有这些复合物的正常细胞相比,没有观察到明显的选择性。值得注意的是,所代表的复合物5a可以促进活性氧水平的增加并通过细胞凋亡诱导细胞死亡。
更新日期:2022-06-23
中文翻译:

基于吡啶-胺配体的铱 (III) 和铑 (III) 胺、亚胺和酰胺配合物的形成:由反应条件、取代基变化和金属中心引起的结构多样性
在此,我们展示了基于吡啶-胺配体的半夹心铱(III)和铑(III)配合物的不同配位模式。通过改变反应条件(氮气/外加氧气氛、反应时间、和溶剂)以及金属和配体的结构变化。总的来说,吡啶-胺配体与[(η 5 -C 5 (CH 3 ) 5 )MCl 2 ] 2的反应(M = Ir 或 Rh)在外来氧存在下提供氧化的吡啶基 - 亚胺复合物。铱(III) 和铑(III) 胺配合物的氧化可能机理通过副产物过氧化氢的检测得到证实。此外,当使用非极性溶剂 CH 2 Cl 2代替 CH 3 OH时,有利于形成吡啶基-胺络合物。很少报道的具有[(η 5 -Cp*)IrCl 3 ] 阴离子的络合物也可以在不添加NH 4 PF 6的情况下获得。空间大体积i的引入配体桥碳上的 -Bu 基团导致形成稳定的 16 电子吡啶基-酰胺络合物。吡啶基-胺铱(III)和铑(III)配合物也是在N 2气氛下合成的,整个过程中没有检测到H 2 O 2 。特别是,还评估了这些复合物对 A549 和 HeLa 人癌细胞的水溶液稳定性和体外细胞毒性。对于癌细胞与具有这些复合物的正常细胞相比,没有观察到明显的选择性。值得注意的是,所代表的复合物5a可以促进活性氧水平的增加并通过细胞凋亡诱导细胞死亡。









































 京公网安备 11010802027423号
京公网安备 11010802027423号