当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synchronous Moderate Oxidation and Adsorption on the Surface of γ-MnO2 for Efficient Iodide Removal from Water
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.est.2c01682 Nan Wang 1 , Gong Zhang 1 , Ruoxi Xiong 1 , Ruiping Liu 1 , Huijuan Liu 1 , Jiuhui Qu 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.est.2c01682 Nan Wang 1 , Gong Zhang 1 , Ruoxi Xiong 1 , Ruiping Liu 1 , Huijuan Liu 1 , Jiuhui Qu 1
Affiliation
|
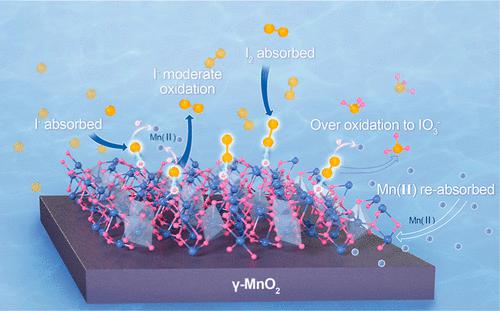
Long-term exposure to excessive iodine via drinking water presents health risks. Moderate oxidation of iodide (I–) to iodine (I2) has a better iodine removal effect than excessive oxidation to iodate (IO3–). This study combines computational and experimental methods to construct a heterogeneous interface with synchronous I– moderate oxidation and I2 adsorption to increase the total iodine removal. Compared to other forms of crystal manganese dioxide (MnO2), theoretical calculations predict that MnO2 with a γ-crystal structure has the lowest adsorption energy, that is, −1.20 eV, and a slight overlap between the conduction and valence bands, which favors electron transfer between I– and Mn(IV) and I2 adsorption. Thus, γ-type MnO2 was designed by adjusting the precursor Mn sources and hydrothermal reaction conditions. The liquid chromatography–inductively coupled plasma–mass spectrometry and high-performance liquid chromatography confirmed that the total iodine concentration in water decreased from 173.7 to 36.3 μg/L after 2 h, with 200 mg/L γ-MnO2 dosage lower than the national standard of 0.1 mg/L. A minute proportion of I– in water was converted to IO3– (approximately 1.1 μg/L). The current I– adsorbent performed better than previously reported ones. During iodine removal, most of the I– migrated from water to the surface of γ-MnO2, and the ratio of I– to I2 was determined to be 1:0.6 by X-ray photoelectron spectroscopy. This study evaluates iodine species transformation and an optimum strategy for heterogeneous interface design; it is promising for treating high-iodine groundwater.
中文翻译:

γ-MnO2 表面同步适度氧化和吸附,有效去除水中的碘化物
长期通过饮用水摄入过量碘会带来健康风险。碘化物(I -)适度氧化成碘(I 2 )比过度氧化成碘酸盐(IO 3 - )具有更好的除碘效果。本研究将计算和实验方法相结合,构建了一个具有同步 I -适度氧化和 I 2吸附的非均相界面,以提高总碘去除率。与其他形式的晶体二氧化锰(MnO 2)相比,理论计算预测 MnO 2γ-晶体结构的吸附能最低,即-1.20 eV,导带和价带之间有轻微的重叠,有利于I-和Mn(IV)之间的电子转移和I 2吸附。因此,通过调整前驱体Mn源和水热反应条件设计了γ型MnO 2 。液相色谱-电感耦合等离子体-质谱和高效液相色谱证实,2 h后水中总碘浓度由173.7降至36.3 μg/L,γ-MnO 2用量低于国家标准200 mg/L标准为 0.1 毫克/升。一小部分 I -在水中被转化为 IO 3 -(约 1.1 微克/升)。目前的 I -吸附剂比以前报道的表现更好。在除碘过程中,大部分I -从水中迁移到γ-MnO 2表面,通过X射线光电子能谱测定I -与I 2的比例为1:0.6。本研究评估了碘物质转化和异质界面设计的最佳策略;有望用于处理高碘地下水。
更新日期:2022-06-23
中文翻译:

γ-MnO2 表面同步适度氧化和吸附,有效去除水中的碘化物
长期通过饮用水摄入过量碘会带来健康风险。碘化物(I -)适度氧化成碘(I 2 )比过度氧化成碘酸盐(IO 3 - )具有更好的除碘效果。本研究将计算和实验方法相结合,构建了一个具有同步 I -适度氧化和 I 2吸附的非均相界面,以提高总碘去除率。与其他形式的晶体二氧化锰(MnO 2)相比,理论计算预测 MnO 2γ-晶体结构的吸附能最低,即-1.20 eV,导带和价带之间有轻微的重叠,有利于I-和Mn(IV)之间的电子转移和I 2吸附。因此,通过调整前驱体Mn源和水热反应条件设计了γ型MnO 2 。液相色谱-电感耦合等离子体-质谱和高效液相色谱证实,2 h后水中总碘浓度由173.7降至36.3 μg/L,γ-MnO 2用量低于国家标准200 mg/L标准为 0.1 毫克/升。一小部分 I -在水中被转化为 IO 3 -(约 1.1 微克/升)。目前的 I -吸附剂比以前报道的表现更好。在除碘过程中,大部分I -从水中迁移到γ-MnO 2表面,通过X射线光电子能谱测定I -与I 2的比例为1:0.6。本研究评估了碘物质转化和异质界面设计的最佳策略;有望用于处理高碘地下水。










































 京公网安备 11010802027423号
京公网安备 11010802027423号