当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pd-Catalyzed Direct Deoxygenative Arylation of Non-π-Extended Benzyl Alcohols with Boronic Acids via Transient Formation of Non-Innocent Isoureas
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-06-23 , DOI: 10.1021/acscatal.2c01858 Georgios Toupalas 1 , Gianin Thomann 1 , Lukas Schlemper 1 , Miguel A. Rivero-Crespo 1 , Hendrik L. Schmitt 1 , Bill Morandi 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-06-23 , DOI: 10.1021/acscatal.2c01858 Georgios Toupalas 1 , Gianin Thomann 1 , Lukas Schlemper 1 , Miguel A. Rivero-Crespo 1 , Hendrik L. Schmitt 1 , Bill Morandi 1
Affiliation
|
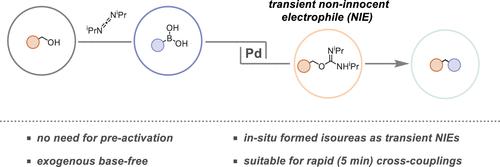
We report the direct arylation of non-derivatized alcohols with boronic acids and demonstrate that a Pd catalyst, in combination with a carbodiimide, can be used to forge a C–C bond via the transient formation of non-innocent isoureas from the corresponding alcohols. Besides further polarizing the C–O bond, the transiently generated isourea contains a masked base that is released during the reaction to enable catalytic turnover under exogenous base-free conditions. The developed concept was benchmarked against the coupling of non-π-extended benzyl alcohols and boronic acids and led to the formation of a C–C bond between differently decorated coupling partners. Notably, the strategic generation of non-innocent isoureas endows this C–O cleavage reaction with high orthogonality over conventional electrophiles and enables the employment of highly base-sensitive boronic acids. Additionally, the preformed isoureas can be leveraged for rapid (5 min reaction time) exogenous base-free coupling reactions, which work under conventional thermal conditions and do not rely on customized catalysts or specialized equipment. The synthetic investigations were also complemented by preliminary mechanistic studies. More broadly, the presented work bridges a conceptual gap between two important research areas, that is, carbodiimide-mediated alcohol activation and deoxygenative transition metal-catalyzed coupling chemistry, providing a promising blueprint for direct catalytic deoxygenative reactions.
中文翻译:

Pd催化非π-扩链苄醇与硼酸通过非无害异脲的瞬时形成直接脱氧芳基化
我们报道了非衍生化醇与硼酸的直接芳基化反应,并证明 Pd 催化剂与碳二亚胺结合可用于通过从相应的醇中瞬时形成非无害异脲来形成 C-C 键。除了进一步极化 C-O 键外,瞬时产生的异脲还包含一个掩蔽的碱基,该碱基在反应过程中释放,以在外源无碱基条件下实现催化转换。所开发的概念以非 π 延伸苯甲醇和硼酸的偶联为基准,并导致在不同修饰的偶联伙伴之间形成 C-C 键。尤其,非无辜异脲的战略性生成赋予了这种 C-O 裂解反应比传统亲电试剂更高的正交性,并能够使用高度碱敏感的硼酸。此外,预成型的异脲可用于快速(5 分钟反应时间)外源性无碱偶联反应,该反应在常规热条件下工作,不依赖定制催化剂或专用设备。综合研究也得到了初步机械研究的补充。更广泛地说,所提出的工作弥合了两个重要研究领域之间的概念差距,即碳二亚胺介导的醇活化和脱氧过渡金属催化的偶联化学,为直接催化脱氧反应提供了有希望的蓝图。此外,预成型的异脲可用于快速(5 分钟反应时间)外源性无碱偶联反应,该反应在常规热条件下工作,不依赖定制催化剂或专用设备。综合研究也得到了初步机械研究的补充。更广泛地说,所提出的工作弥合了两个重要研究领域之间的概念差距,即碳二亚胺介导的醇活化和脱氧过渡金属催化的偶联化学,为直接催化脱氧反应提供了有希望的蓝图。此外,预成型的异脲可用于快速(5 分钟反应时间)外源性无碱偶联反应,该反应在常规热条件下工作,不依赖定制催化剂或专用设备。综合研究也得到了初步机械研究的补充。更广泛地说,所提出的工作弥合了两个重要研究领域之间的概念差距,即碳二亚胺介导的醇活化和脱氧过渡金属催化的偶联化学,为直接催化脱氧反应提供了有希望的蓝图。综合研究也得到了初步机械研究的补充。更广泛地说,所提出的工作弥合了两个重要研究领域之间的概念差距,即碳二亚胺介导的醇活化和脱氧过渡金属催化的偶联化学,为直接催化脱氧反应提供了有希望的蓝图。综合研究也得到了初步机械研究的补充。更广泛地说,所提出的工作弥合了两个重要研究领域之间的概念差距,即碳二亚胺介导的醇活化和脱氧过渡金属催化的偶联化学,为直接催化脱氧反应提供了有希望的蓝图。
更新日期:2022-06-23
中文翻译:

Pd催化非π-扩链苄醇与硼酸通过非无害异脲的瞬时形成直接脱氧芳基化
我们报道了非衍生化醇与硼酸的直接芳基化反应,并证明 Pd 催化剂与碳二亚胺结合可用于通过从相应的醇中瞬时形成非无害异脲来形成 C-C 键。除了进一步极化 C-O 键外,瞬时产生的异脲还包含一个掩蔽的碱基,该碱基在反应过程中释放,以在外源无碱基条件下实现催化转换。所开发的概念以非 π 延伸苯甲醇和硼酸的偶联为基准,并导致在不同修饰的偶联伙伴之间形成 C-C 键。尤其,非无辜异脲的战略性生成赋予了这种 C-O 裂解反应比传统亲电试剂更高的正交性,并能够使用高度碱敏感的硼酸。此外,预成型的异脲可用于快速(5 分钟反应时间)外源性无碱偶联反应,该反应在常规热条件下工作,不依赖定制催化剂或专用设备。综合研究也得到了初步机械研究的补充。更广泛地说,所提出的工作弥合了两个重要研究领域之间的概念差距,即碳二亚胺介导的醇活化和脱氧过渡金属催化的偶联化学,为直接催化脱氧反应提供了有希望的蓝图。此外,预成型的异脲可用于快速(5 分钟反应时间)外源性无碱偶联反应,该反应在常规热条件下工作,不依赖定制催化剂或专用设备。综合研究也得到了初步机械研究的补充。更广泛地说,所提出的工作弥合了两个重要研究领域之间的概念差距,即碳二亚胺介导的醇活化和脱氧过渡金属催化的偶联化学,为直接催化脱氧反应提供了有希望的蓝图。此外,预成型的异脲可用于快速(5 分钟反应时间)外源性无碱偶联反应,该反应在常规热条件下工作,不依赖定制催化剂或专用设备。综合研究也得到了初步机械研究的补充。更广泛地说,所提出的工作弥合了两个重要研究领域之间的概念差距,即碳二亚胺介导的醇活化和脱氧过渡金属催化的偶联化学,为直接催化脱氧反应提供了有希望的蓝图。综合研究也得到了初步机械研究的补充。更广泛地说,所提出的工作弥合了两个重要研究领域之间的概念差距,即碳二亚胺介导的醇活化和脱氧过渡金属催化的偶联化学,为直接催化脱氧反应提供了有希望的蓝图。综合研究也得到了初步机械研究的补充。更广泛地说,所提出的工作弥合了两个重要研究领域之间的概念差距,即碳二亚胺介导的醇活化和脱氧过渡金属催化的偶联化学,为直接催化脱氧反应提供了有希望的蓝图。







































 京公网安备 11010802027423号
京公网安备 11010802027423号