当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing rates
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-06-23 , DOI: 10.1039/d2cp01412b Marco Simoni 1 , Theodore Hanein 1 , Chun Long Woo 1 , Mark Tyrer 2 , Magnus Nyberg 3 , Juan-Carlos Martinez 3 , Nestor I Quintero-Mora 4 , John L Provis 1 , Hajime Kinoshita 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-06-23 , DOI: 10.1039/d2cp01412b Marco Simoni 1 , Theodore Hanein 1 , Chun Long Woo 1 , Mark Tyrer 2 , Magnus Nyberg 3 , Juan-Carlos Martinez 3 , Nestor I Quintero-Mora 4 , John L Provis 1 , Hajime Kinoshita 1
Affiliation
|
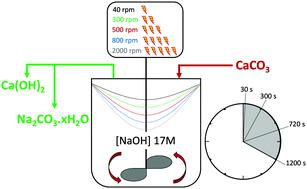
The decarbonisation of CaCO3 is essential for the production of lime (Ca(OH)2 and CaO), which is a commodity required in several large industries and the main precursor for cement production. CaCO3 is usually decarbonised at high temperatures, generating gaseous CO2 which will require post-process capture to minimise its release into the environment. We have developed a new process that can decarbonise CaCO3 under ambient conditions, while sequestering the CO2 as Na2CO3·H2O or Na2CO3 in the same stage. Here, the effects of increasing stirring rates and residence times on reaction efficiency of the key reaction occurring between CaCO3 and NaOH solution are studied. It is shown that the reaction is enhanced at lower stirring rates and longer residence times up to 300 seconds of contact between the reactants. The mass balance performed for Ca and CO2 revealed that up to the 95% of the process CO2 embodied in CaCO3 was sequestered, with maximum capture rate assessed at nn moles CO2 captured per second of reaction progress. A deeper insight into the precipitation of Na2CO3·H2O or Na2CO3 under different reaction conditions was gained, and SEM-EDX analysis enabled the observation of the reaction front by detection of Na migrating towards inner regions of partially-reacted limestone chalk particles.
中文翻译:

环境条件下氢氧化钠溶液中碳酸钙的脱碳:停留时间和混合速率的影响
CaCO 3的脱碳对于生产石灰(Ca(OH) 2和 CaO)至关重要,石灰是几个大型行业所需的商品,也是水泥生产的主要前体。CaCO 3通常在高温下脱碳,产生气态 CO 2,这将需要后处理捕获以尽量减少其释放到环境中。我们开发了一种新工艺,可以在环境条件下使 CaCO 3脱碳,同时将 CO 2隔离为 Na 2 CO 3 ·H 2 O 或 Na 2 CO 3在同一阶段。在这里,研究了增加搅拌速率和停留时间对 CaCO 3和 NaOH 溶液之间发生的关键反应的反应效率的影响。结果表明,在较低的搅拌速率和较长的停留时间下,反应物之间的接触时间最长可达 300 秒,反应会增强。对 Ca 和 CO 2进行的质量平衡表明,在 CaCO 3中体现的过程中高达 95% 的 CO 2被隔离,最大捕获率评估为反应进程每秒捕获nn摩尔 CO 2 。深入了解 Na 2 CO 3 ·H 2 O 或 Na的沉淀获得了不同反应条件下的2 CO 3,SEM-EDX分析通过检测Na向部分反应的石灰石白垩颗粒内部区域迁移来观察反应前沿。
更新日期:2022-06-24
中文翻译:

环境条件下氢氧化钠溶液中碳酸钙的脱碳:停留时间和混合速率的影响
CaCO 3的脱碳对于生产石灰(Ca(OH) 2和 CaO)至关重要,石灰是几个大型行业所需的商品,也是水泥生产的主要前体。CaCO 3通常在高温下脱碳,产生气态 CO 2,这将需要后处理捕获以尽量减少其释放到环境中。我们开发了一种新工艺,可以在环境条件下使 CaCO 3脱碳,同时将 CO 2隔离为 Na 2 CO 3 ·H 2 O 或 Na 2 CO 3在同一阶段。在这里,研究了增加搅拌速率和停留时间对 CaCO 3和 NaOH 溶液之间发生的关键反应的反应效率的影响。结果表明,在较低的搅拌速率和较长的停留时间下,反应物之间的接触时间最长可达 300 秒,反应会增强。对 Ca 和 CO 2进行的质量平衡表明,在 CaCO 3中体现的过程中高达 95% 的 CO 2被隔离,最大捕获率评估为反应进程每秒捕获nn摩尔 CO 2 。深入了解 Na 2 CO 3 ·H 2 O 或 Na的沉淀获得了不同反应条件下的2 CO 3,SEM-EDX分析通过检测Na向部分反应的石灰石白垩颗粒内部区域迁移来观察反应前沿。











































 京公网安备 11010802027423号
京公网安备 11010802027423号