当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Separation and Collision Cross Section Measurements of Protein Complexes Afforded by a Modular Drift Tube Coupled to an Orbitrap Mass Spectrometer
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.analchem.2c01653 Sarah N Sipe 1 , James D Sanders 1 , Tobias Reinecke 2 , Brian H Clowers 2 , Jennifer S Brodbelt 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2022-06-23 , DOI: 10.1021/acs.analchem.2c01653 Sarah N Sipe 1 , James D Sanders 1 , Tobias Reinecke 2 , Brian H Clowers 2 , Jennifer S Brodbelt 1
Affiliation
|
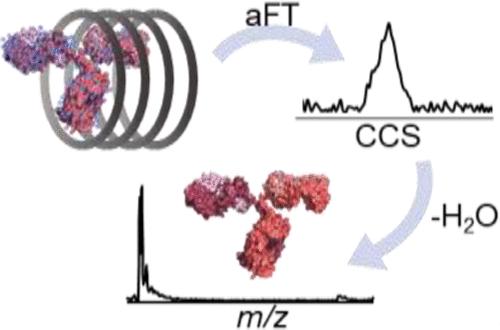
New developments in analytical technologies and biophysical methods have advanced the characterization of increasingly complex biomolecular assemblies using native mass spectrometry (MS). Ion mobility methods, in particular, have enabled a new dimension of structural information and analysis of proteins, allowing separation of conformations and providing size and shape insights based on collision cross sections (CCSs). Based on the concepts of absorption-mode Fourier transform (aFT) multiplexing ion mobility spectrometry (IMS), here, a modular drift tube design proves capable of separating native-like proteins up to 148 kDa with resolution up to 45. Coupled with high-resolution Orbitrap MS, binding of small ligands and cofactors can be resolved in the mass domain and correlated to changes in structural heterogeneity observed in the ion-neutral CCS distributions. We also demonstrate the ability to rapidly determine accurate CCSs for proteins with 1-min aFT-IMS-MS sweeps without the need for calibrants or correction factors.
中文翻译:

由与 Orbitrap 质谱仪耦合的模块化漂移管提供的蛋白质复合物的分离和碰撞截面测量
分析技术和生物物理方法的新发展促进了使用原生质谱 (MS) 来表征日益复杂的生物分子组装体。特别是离子淌度方法,使结构信息和蛋白质分析进入了新的维度,允许分离构象并提供基于碰撞截面 (CCS) 的尺寸和形状见解。基于吸收模式傅里叶变换 (aFT) 多重离子迁移谱 (IMS) 的概念,模块化漂移管设计证明能够分离高达 148 kDa 的类天然蛋白质,分辨率高达 45。分辨率 Orbitrap MS,小配体和辅因子的结合可以在质量域中解析,并与离子中性 CCS 分布中观察到的结构异质性变化相关。我们还展示了通过 1 分钟 aFT-IMS-MS 扫描快速确定蛋白质的准确 CCS 的能力,无需校准物或校正因子。
更新日期:2022-06-23
中文翻译:

由与 Orbitrap 质谱仪耦合的模块化漂移管提供的蛋白质复合物的分离和碰撞截面测量
分析技术和生物物理方法的新发展促进了使用原生质谱 (MS) 来表征日益复杂的生物分子组装体。特别是离子淌度方法,使结构信息和蛋白质分析进入了新的维度,允许分离构象并提供基于碰撞截面 (CCS) 的尺寸和形状见解。基于吸收模式傅里叶变换 (aFT) 多重离子迁移谱 (IMS) 的概念,模块化漂移管设计证明能够分离高达 148 kDa 的类天然蛋白质,分辨率高达 45。分辨率 Orbitrap MS,小配体和辅因子的结合可以在质量域中解析,并与离子中性 CCS 分布中观察到的结构异质性变化相关。我们还展示了通过 1 分钟 aFT-IMS-MS 扫描快速确定蛋白质的准确 CCS 的能力,无需校准物或校正因子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号