当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron(III)-Catalyzed Difluoroalkylation of Aryl Alkynes with Difluoroenol Silyl Ether in the Presence of Trimethylsilyl Chloride
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-06-22 , DOI: 10.1002/adsc.202200277 Meng-Meng Guo 1 , Xuan-Di Song 1 , Xiang Liu 1 , Ya-Wen Zheng 1 , Xue-Qiang Chu 1 , Weidong Rao 2 , Zhi-Liang Shen 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-06-22 , DOI: 10.1002/adsc.202200277 Meng-Meng Guo 1 , Xuan-Di Song 1 , Xiang Liu 1 , Ya-Wen Zheng 1 , Xue-Qiang Chu 1 , Weidong Rao 2 , Zhi-Liang Shen 1
Affiliation
|
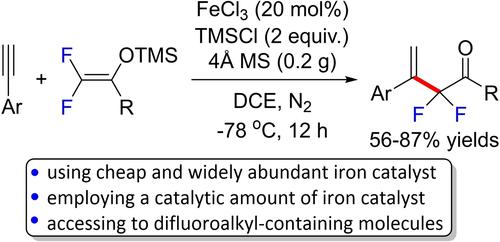
The reaction of non-fluorinated silyl enol ether with alkyne is useful for the synthesis of β,γ-unsaturated carbonyl compounds. However, most of the existing methods for realizing such organic transformation usually employ stoichiometric amounts of relatively expensive Lewis acids/metallic salts. Herein, an iron(III)-catalyzed difluoroalkylation of aryl alkynes with difluoroenol silyl ether was developed. The reactions proceeded smoothly in the presence of a catalytic amount of iron(III) chloride, stoichiometric amounts of trimethylsilyl chloride, and 4 Å molecular sieves in dichloroethane (DCE) to afford the corresponding α-alkenyl-α,α-difluoroketones in modest to good yields. Remarkably, among the various metallic salts screened, cheap and less-toxic iron(III) salt was found to be the most efficient Lewis acid catalyst for the present reaction. In addition, in the absence of iron(III) chloride or trimethylsilyl chloride, either no reaction occurred or considerably reduced reaction performance was observed. Moreover, the use of Brønsted acid to replace iron(III) chloride as reaction catalyst failed to promote the reaction. The reaction could be scaled up and the obtained difluoroalkylated carbonyl compound serves as a versatile building block which could be subjected to late-stage diversification to be converted into useful organic molecules containing CF2H and CF2CF2 moieties. Deuterated experiments showed that the proton in the generated alkene product should originate from trace amounts of water present in the reaction system.
中文翻译:

三甲基氯硅烷存在下铁 (III) 催化芳基炔烃与二氟烯醇甲硅烷基醚的二氟烷基化反应
非氟化甲硅烷基烯醇醚与炔烃的反应可用于合成β,γ-不饱和羰基化合物。然而,大多数实现这种有机转化的现有方法通常使用化学计量量的相对昂贵的路易斯酸/金属盐。在此,开发了铁 (III) 催化的芳基炔烃与二氟烯醇甲硅烷基醚的二氟烷基化反应。在催化量的氯化铁 (III)、化学计量的三甲基氯硅烷和 4 Å的存在下,反应顺利进行分子筛在二氯乙烷 (DCE) 中以适度至良好的收率提供相应的 α-烯基-α,α-二氟酮。值得注意的是,在筛选的各种金属盐中,发现廉价且毒性较小的铁 (III) 盐是本反应最有效的路易斯酸催化剂。此外,在不存在氯化铁(III)或氯化三甲基甲硅烷的情况下,没有反应发生或观察到反应性能显着降低。此外,使用布朗斯台德酸代替氯化铁(III)作为反应催化剂未能促进反应。该反应可以扩大规模,并且获得的二氟烷基化羰基化合物可作为通用结构单元,可进行后期多样化以转化为有用的含有 CF 2的有机分子H和CF 2 CF 2部分。氘代实验表明,生成的烯烃产物中的质子应该来源于反应体系中存在的痕量水。
更新日期:2022-06-22
中文翻译:

三甲基氯硅烷存在下铁 (III) 催化芳基炔烃与二氟烯醇甲硅烷基醚的二氟烷基化反应
非氟化甲硅烷基烯醇醚与炔烃的反应可用于合成β,γ-不饱和羰基化合物。然而,大多数实现这种有机转化的现有方法通常使用化学计量量的相对昂贵的路易斯酸/金属盐。在此,开发了铁 (III) 催化的芳基炔烃与二氟烯醇甲硅烷基醚的二氟烷基化反应。在催化量的氯化铁 (III)、化学计量的三甲基氯硅烷和 4 Å的存在下,反应顺利进行分子筛在二氯乙烷 (DCE) 中以适度至良好的收率提供相应的 α-烯基-α,α-二氟酮。值得注意的是,在筛选的各种金属盐中,发现廉价且毒性较小的铁 (III) 盐是本反应最有效的路易斯酸催化剂。此外,在不存在氯化铁(III)或氯化三甲基甲硅烷的情况下,没有反应发生或观察到反应性能显着降低。此外,使用布朗斯台德酸代替氯化铁(III)作为反应催化剂未能促进反应。该反应可以扩大规模,并且获得的二氟烷基化羰基化合物可作为通用结构单元,可进行后期多样化以转化为有用的含有 CF 2的有机分子H和CF 2 CF 2部分。氘代实验表明,生成的烯烃产物中的质子应该来源于反应体系中存在的痕量水。











































 京公网安备 11010802027423号
京公网安备 11010802027423号