当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bromide-Catalyzed Electrochemical Csp3−H Oxidation of Acetonitrile: Stereoselective Synthesis of Heteroaryl Vinyl Sulfides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-06-22 , DOI: 10.1002/adsc.202200247 Jin-Lin Wan 1 , Jing-Mei Huang 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-06-22 , DOI: 10.1002/adsc.202200247 Jin-Lin Wan 1 , Jing-Mei Huang 2
Affiliation
|
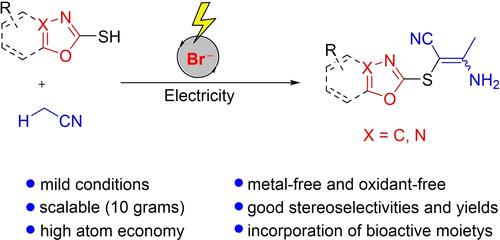
An electrochemical oxidative C−H/S−H cross-coupling reaction between acetonitrile and heteroaryl thiols has been developed. Me4NBr is employed as a redox catalyst to oxidize both the Csp3−H of acetonitrile and S−H of heteroaryl thiols. Heteroaryl vinyl sulfides were afforded under metal-free and oxidant-free reaction conditions in good yields and stereoselectivities with excellent functional group tolerance. The synthetic applicability of the electrochemical method was further highlighted by its easy scalability.
中文翻译:

乙腈的溴化物催化电化学 Csp3−H 氧化:杂芳基乙烯基硫化物的立体选择性合成
已经开发了乙腈和杂芳基硫醇之间的电化学氧化 C-H/S-H 交叉偶联反应。Me 4 NBr 用作氧化还原催化剂以氧化乙腈的 C sp 3 -H 和杂芳基硫醇的 S-H。在无金属和无氧化剂的反应条件下,杂芳基乙烯基硫化物具有良好的收率和立体选择性,具有优异的官能团耐受性。电化学方法的合成适用性因其易于扩展而进一步突出。
更新日期:2022-06-22
中文翻译:

乙腈的溴化物催化电化学 Csp3−H 氧化:杂芳基乙烯基硫化物的立体选择性合成
已经开发了乙腈和杂芳基硫醇之间的电化学氧化 C-H/S-H 交叉偶联反应。Me 4 NBr 用作氧化还原催化剂以氧化乙腈的 C sp 3 -H 和杂芳基硫醇的 S-H。在无金属和无氧化剂的反应条件下,杂芳基乙烯基硫化物具有良好的收率和立体选择性,具有优异的官能团耐受性。电化学方法的合成适用性因其易于扩展而进一步突出。











































 京公网安备 11010802027423号
京公网安备 11010802027423号