当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Flexible Approach for the Synthesis of Annulated 4H-Pyrans Based on a Cu(I)-Catalyzed C-Allylation/O-Vinylation Reaction of Cyclic 1-Bromoallyl Tosylates with Cyclic and Acyclic 1,3-Dicarbonyls
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.joc.1c02997 Mehran Khoshbakhsh Foumani 1 , Jürgen Conrad 1 , Wolfgang Frey 2 , Uwe Beifuss 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.joc.1c02997 Mehran Khoshbakhsh Foumani 1 , Jürgen Conrad 1 , Wolfgang Frey 2 , Uwe Beifuss 1
Affiliation
|
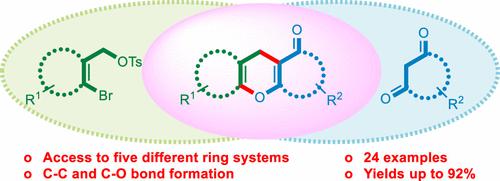
The Cu(I)-catalyzed reaction between five-, six-, seven-, and eight-membered cyclic 1-bromoallyl tosylates and five- and six-membered cyclic 1,3-dicarbonyls in DMF at 80 °C using Cs2CO3 as a base and 2-picolinic acid as an additive selectively delivers a wide array of bisannulated 4H-pyrans in a single step with yields up to 92%. The transformations are considered to proceed as intermolecular C-allylations/intramolecular O-vinylations. With six-membered cyclic 1-bromoallyl tosylates and acyclic β-ketoesters as substrates, the corresponding 5,6,7,8-tetrahydro-4H-chromene-3-carboxylates are obtained with yields up to 59%.
中文翻译:

基于 Cu(I) 催化的环状 1-溴代烯丙基甲苯磺酸酯与环状和非环状 1,3-二羰基化合物的 C-烯丙基化/O-乙烯基化反应合成环化 4H-吡喃的灵活方法
使用 Cs 2 CO在 DMF 中于 80 °C 下 Cu(I) 催化的五元、六元、七元和八元环状 1-溴代烯丙基甲苯磺酸酯与五元和六元环状 1,3-二羰基之间的反应3作为碱和 2-吡啶甲酸作为添加剂在一个步骤中选择性地提供广泛的双环化 4 H-吡喃,产率高达 92%。这些转化被认为是作为分子间 C-烯丙基化/分子内 O-乙烯基化进行的。以六元环状 1-溴代烯丙基甲苯磺酸酯和无环 β-酮酯为底物,得到相应的 5,6,7,8-四氢-4 H-色烯-3-羧酸盐,产率高达 59%。
更新日期:2022-06-22
中文翻译:

基于 Cu(I) 催化的环状 1-溴代烯丙基甲苯磺酸酯与环状和非环状 1,3-二羰基化合物的 C-烯丙基化/O-乙烯基化反应合成环化 4H-吡喃的灵活方法
使用 Cs 2 CO在 DMF 中于 80 °C 下 Cu(I) 催化的五元、六元、七元和八元环状 1-溴代烯丙基甲苯磺酸酯与五元和六元环状 1,3-二羰基之间的反应3作为碱和 2-吡啶甲酸作为添加剂在一个步骤中选择性地提供广泛的双环化 4 H-吡喃,产率高达 92%。这些转化被认为是作为分子间 C-烯丙基化/分子内 O-乙烯基化进行的。以六元环状 1-溴代烯丙基甲苯磺酸酯和无环 β-酮酯为底物,得到相应的 5,6,7,8-四氢-4 H-色烯-3-羧酸盐,产率高达 59%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号