当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective C3-Allylation and Formal [3 + 2]-Annulation of Spiro-Aziridine Oxindoles: Synthesis of 5′-Substituted Spiro[pyrrolidine-3,3′-oxindoles] and Coerulescine
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.joc.2c00863 Sk Abu Saleh 1, 2 , Atanu Hazra 1, 2 , Maya Shankar Singh 2 , Saumen Hajra 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.joc.2c00863 Sk Abu Saleh 1, 2 , Atanu Hazra 1, 2 , Maya Shankar Singh 2 , Saumen Hajra 1
Affiliation
|
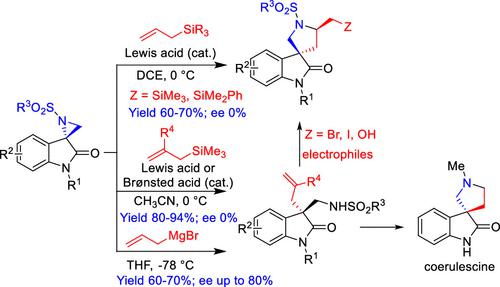
Brønsted acid- and/or Lewis acid-catalyzed selective C3-allylation and formal [3 + 2]-annulation of spiro-aziridine oxindoles with allylsilanes have been demonstrated to deliver direct access to 3-allyl-3-aminomethyl oxindoles and 5-silyl methyl spiro[pyrrolidine-3,3′-oxindoles], respectively. The acid-catalyzed methods do not provide any stereoselectivity when chiral spiroaziridines are used. However, the reaction of nonracemic sprioaziridines with allyl-Grignard reagent under catalyst-free conditions afforded 3-allyl-3-aminomethyl oxindoles with good stereoselectivity (ee up to 80%). The allylation protocol is utilized for the short synthesis of coerulescine and various 5′-substituted spiro[pyrrolidine-3,3′-oxindoles].
中文翻译:

螺-氮丙啶羟吲哚的选择性 C3-烯丙基化和形式 [3 + 2]-环化:5'-取代螺[吡咯烷-3,3'-羟吲哚] 和 Coerulescine 的合成
布朗斯台德酸和/或路易斯酸催化的选择性 C3 烯丙基化和形式 [3 + 2] 环化螺环氮丙啶羟吲哚与烯丙基硅烷已被证明可直接获得 3-烯丙基-3-氨基甲基羟吲哚和 5-甲硅烷基甲基螺[吡咯烷-3,3'-羟吲哚],分别。当使用手性螺氮丙啶时,酸催化方法不提供任何立体选择性。然而,非外消旋螺氮丙啶与烯丙基-格氏试剂在无催化剂条件下的反应提供了具有良好立体选择性(ee 高达 80%)的 3-烯丙基-3-氨基甲基羟吲哚。烯丙基化方案用于短期合成 coerulescine 和各种 5'-取代螺 [pyrrolidine-3,3'-oxindoles]。
更新日期:2022-06-22
中文翻译:

螺-氮丙啶羟吲哚的选择性 C3-烯丙基化和形式 [3 + 2]-环化:5'-取代螺[吡咯烷-3,3'-羟吲哚] 和 Coerulescine 的合成
布朗斯台德酸和/或路易斯酸催化的选择性 C3 烯丙基化和形式 [3 + 2] 环化螺环氮丙啶羟吲哚与烯丙基硅烷已被证明可直接获得 3-烯丙基-3-氨基甲基羟吲哚和 5-甲硅烷基甲基螺[吡咯烷-3,3'-羟吲哚],分别。当使用手性螺氮丙啶时,酸催化方法不提供任何立体选择性。然而,非外消旋螺氮丙啶与烯丙基-格氏试剂在无催化剂条件下的反应提供了具有良好立体选择性(ee 高达 80%)的 3-烯丙基-3-氨基甲基羟吲哚。烯丙基化方案用于短期合成 coerulescine 和各种 5'-取代螺 [pyrrolidine-3,3'-oxindoles]。









































 京公网安备 11010802027423号
京公网安备 11010802027423号