当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Pot Synthesis of Multifunctionalized 1-Pyrrolines from 2-Alkyl-2H-azirines and Diazocarbonyl Compounds
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.joc.2c00977 Ilya P Filippov 1 , Mikhail S Novikov 1 , Alexander F Khlebnikov 1 , Nikolai V Rostovskii 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-06-22 , DOI: 10.1021/acs.joc.2c00977 Ilya P Filippov 1 , Mikhail S Novikov 1 , Alexander F Khlebnikov 1 , Nikolai V Rostovskii 1
Affiliation
|
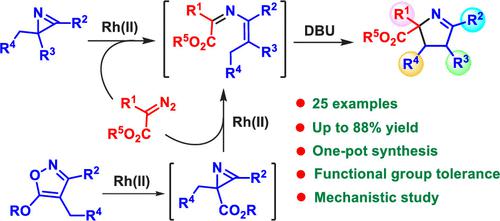
A novel strategy for the synthesis of 1-pyrrolines based on formal [4 + 1] annulation of 2-alkyl-2H-azirines with diazocarbonyl compounds has been developed. This one-pot approach includes the Rh(II)-catalyzed formation of 4-alkyl-2-azabuta-1,3-dienes, followed by the DBU-promoted cyclization, and features a good substrate tolerance. The 1-pyrrolines containing an ester group at the C3 were prepared in a three-step one-pot procedure starting from 5-alkoxyisoxazoles. The cyclization of 2-azabutadienes to 1-pyrrolines most likely proceeds via the 6π electrocyclization of a conjugated NH-azomethine ylide.
中文翻译:

从 2-烷基-2H-氮杂环丙烷和重氮羰基化合物一锅法合成多功能化 1-吡咯啉
已经开发了一种基于 2-烷基-2 H-氮丙啶与重氮羰基化合物的形式 [4 + 1] 环化合成 1-吡咯啉的新策略。这种一锅法包括 Rh(II) 催化的 4-烷基-2-azabuta-1,3-二烯的形成,然后是 DBU 促进的环化,并且具有良好的底物耐受性。在 C3 处含有酯基的 1-吡咯啉是由 5-烷氧基异恶唑开始的三步一锅法制备的。2-氮杂丁二烯环化为 1-吡咯啉很可能通过共轭的 NH-偶氮甲碱叶立德的 6π 电环化进行。
更新日期:2022-06-22
中文翻译:

从 2-烷基-2H-氮杂环丙烷和重氮羰基化合物一锅法合成多功能化 1-吡咯啉
已经开发了一种基于 2-烷基-2 H-氮丙啶与重氮羰基化合物的形式 [4 + 1] 环化合成 1-吡咯啉的新策略。这种一锅法包括 Rh(II) 催化的 4-烷基-2-azabuta-1,3-二烯的形成,然后是 DBU 促进的环化,并且具有良好的底物耐受性。在 C3 处含有酯基的 1-吡咯啉是由 5-烷氧基异恶唑开始的三步一锅法制备的。2-氮杂丁二烯环化为 1-吡咯啉很可能通过共轭的 NH-偶氮甲碱叶立德的 6π 电环化进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号