当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydroaminoalkylation for the Catalytic Addition of Amines to Alkenes or Alkynes: Diverse Mechanisms Enable Diverse Substrate Scope
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-06-22 , DOI: 10.1021/jacs.1c10397 Rebecca C DiPucchio 1 , Sorin-Claudiu Rosca 1 , Laurel L Schafer 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-06-22 , DOI: 10.1021/jacs.1c10397 Rebecca C DiPucchio 1 , Sorin-Claudiu Rosca 1 , Laurel L Schafer 1
Affiliation
|
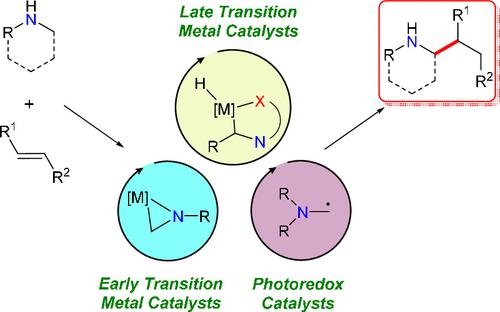
Hydroaminoalkylation is a powerful, atom-economic catalytic reaction for the reaction of amines with alkenes and alkynes. This C–H functionalization reaction allows for the atom-economic alkylation of amines using simple alkenes or alkynes as the alkylating agents. This transformation has significant potential for transformative approaches in the pharmaceutical, agrochemical, and fine chemical industries in the preparation of selectively substituted amines and N-heterocycles and shows promise in materials science for the synthesis of functional and responsive aminated materials. Different early transition-metal, late transition-metal, and photoredox catalysts mediate hydroaminoalkylation by distinct mechanistic pathways. These mechanistic insights have resulted in the development of new catalysts and reaction conditions to realize hydroaminoalkylation with a broad range of substrates: activated and unactivated, terminal and internal, C–C double and triple bonds with aryl or alkyl primary, secondary, or tertiary amines, including N-heterocyclic amines. By deploying select catalysts with specific substrate combinations, control over regioselectivity, diastereoselectivity, and enantioselectivity has been realized. Key barriers to widespread adoption of this reaction include air and moisture sensitivity for early transition-metal catalysts as well as a heavy dependence on amine protecting or directing groups for late transition-metal or photocatalytic routes. Advances in improved catalyst robustness, substrate scope, and regio-/stereoselective reactions with early- and late transition-metal catalysts, as well as photoredox catalysis, are highlighted, and opportunities for further catalyst and reaction development are included. This perspective shows that hydroaminoalkylation has the potential to be a disruptive and transformative strategy for the synthesis of selectively substituted amines and N-heterocycles from simple amines and alkenes.
中文翻译:

用于将胺催化加成烯烃或炔烃的氢化氨基烷基化:多种机制可实现多种底物范围
氢氨基烷基化是一种强大的原子经济催化反应,用于胺与烯烃和炔烃的反应。这种 C-H 官能化反应允许使用简单的烯烃或炔烃作为烷基化剂对胺进行原子经济烷基化。这种转变对于制药、农化和精细化工行业在制备选择性取代胺和N-杂环,并在材料科学中显示出合成功能性和响应性胺化材料的前景。不同的早期过渡金属、晚期过渡金属和光氧化还原催化剂通过不同的机理途径介导氢化氨基烷基化。这些机理见解导致开发了新的催化剂和反应条件,以实现具有广泛底物的氢化氨基烷基化:活化和未活化、末端和内部、C-C 双键和三键与芳基或烷基伯胺、仲胺或叔胺, 包括N-杂环胺。通过部署具有特定底物组合的选择催化剂,实现了对区域选择性、非对映选择性和对映选择性的控制。广泛采用该反应的主要障碍包括早期过渡金属催化剂对空气和水分的敏感性,以及晚期过渡金属或光催化路线对胺保护或导向基团的严重依赖。重点介绍了在改进催化剂稳定性、底物范围和与早期和晚期过渡金属催化剂的区域/立体选择性反应以及光氧化还原催化方面取得的进展,并包括进一步开发催化剂和反应的机会。来自简单胺和烯烃的N-杂环。
更新日期:2022-06-22
中文翻译:

用于将胺催化加成烯烃或炔烃的氢化氨基烷基化:多种机制可实现多种底物范围
氢氨基烷基化是一种强大的原子经济催化反应,用于胺与烯烃和炔烃的反应。这种 C-H 官能化反应允许使用简单的烯烃或炔烃作为烷基化剂对胺进行原子经济烷基化。这种转变对于制药、农化和精细化工行业在制备选择性取代胺和N-杂环,并在材料科学中显示出合成功能性和响应性胺化材料的前景。不同的早期过渡金属、晚期过渡金属和光氧化还原催化剂通过不同的机理途径介导氢化氨基烷基化。这些机理见解导致开发了新的催化剂和反应条件,以实现具有广泛底物的氢化氨基烷基化:活化和未活化、末端和内部、C-C 双键和三键与芳基或烷基伯胺、仲胺或叔胺, 包括N-杂环胺。通过部署具有特定底物组合的选择催化剂,实现了对区域选择性、非对映选择性和对映选择性的控制。广泛采用该反应的主要障碍包括早期过渡金属催化剂对空气和水分的敏感性,以及晚期过渡金属或光催化路线对胺保护或导向基团的严重依赖。重点介绍了在改进催化剂稳定性、底物范围和与早期和晚期过渡金属催化剂的区域/立体选择性反应以及光氧化还原催化方面取得的进展,并包括进一步开发催化剂和反应的机会。来自简单胺和烯烃的N-杂环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号