Water Research ( IF 11.4 ) Pub Date : 2022-06-22 , DOI: 10.1016/j.watres.2022.118786 Shao-Wei Tsai , Dinh Viet Cuong , Chia-Hung Hou
|
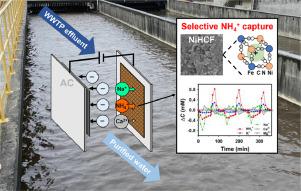
Currently, intercalation materials such as Prussian blue analogs have attracted considerable attention in water treatment applications due to their excellent size-based selectivity toward cations. This study aimed to explore the feasibility of using a nickel hexacyanoferrate (NiHCF) electrode for selective NH4+ capture from effluent from a municipal wastewater treatment plant. To assess the competitive intercalation between NH4+ and other common cations (Na+, Ca2+), a NiHCF//activated carbon (AC) hybrid capacitive deionization (CDI) cell was established to treat mixed-salt solutions. The results of cyclic voltammetry (CV) analysis showed a higher current response of the NiHCF electrode toward NH4+ ions than toward Na+ and Ca2+ ions. In a single-salt solution with NH4+, the optimized operating voltage of the hybrid CDI cell was 0.8 V, with a higher salt adsorption capacity (51.2 mg/g) than those obtained at other voltages (0.1, 0.4, 1.2 V). In a multisalt solution containing NH4+, Na+, and Ca2+ ions, the selectivity coefficients of NH4+/Ca2+ and NH4+/Na+ were 9.5 and 4.9, respectively. The feasibility of selective NH4+ capture using the NiHCF electrode in a hybrid CDI cell was demonstrated by treating the effluent from a municipal wastewater treatment plant (WWTP). The intercalation preference of the NiHCF electrode with the WWTP effluent was NH4+>K+>Na+>Ca2+>Mg2+, and NH4+ showed the highest salt adsorption capacity among the cations during consecutive cycles. Our results revealed that cations with smaller hydrated radii and lower (de)hydration energies were more favorably intercalated by the NiHCF electrode. The results provide important knowledge regarding the use of intercalation-type electrodes for selective nutrient removal and recovery from wastewater.
中文翻译:

使用六氰基铁酸镍电极从市政污水处理厂流出物中选择性捕获铵离子
目前,诸如普鲁士蓝类似物的插层材料由于其对阳离子具有优异的基于尺寸的选择性而在水处理应用中引起了相当大的关注。本研究旨在探讨使用六氰基铁酸镍 (NiHCF) 电极从市政污水处理厂的流出物中选择性捕获NH 4 +的可行性。为了评估NH 4 +和其他常见阳离子(Na +、Ca 2+ )之间的竞争性插层,建立了NiHCF//活性炭(AC)混合电容去离子(CDI)电池来处理混合盐溶液。循环伏安法 (CV) 分析结果表明 NiHCF 电极对 NH 的电流响应更高4 +离子比朝向Na +和Ca 2+离子。在含NH 4 +的单盐溶液中,混合CDI电池的优化工作电压为0.8 V,与其他电压(0.1、0.4、1.2 V)相比具有更高的盐吸附容量(51.2 mg/g) . 在含有NH 4 +、Na +和Ca 2+离子的多盐溶液中,NH 4 + /Ca 2+和NH 4 + /Na +的选择性系数分别为9.5和4.9。选择性NH 4 +的可行性通过处理市政污水处理厂 (WWTP) 的流出物,证明了在混合 CDI 电池中使用 NiHCF 电极进行捕获。NiHCF电极与污水处理厂出水的插层偏好为NH 4 + >K + >Na + >Ca 2+ >Mg 2+,在连续循环中,NH 4 +在阳离子中表现出最高的盐吸附容量。我们的结果表明,具有较小水合半径和较低(脱)水合能的阳离子更容易被 NiHCF 电极插层。该结果提供了有关使用插层型电极从废水中选择性去除和回收养分的重要知识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号