Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2022-06-22 , DOI: 10.1016/j.jhazmat.2022.129428 Jiamin Mai 1 , Tao Yang 1 , Jun Ma 2
|
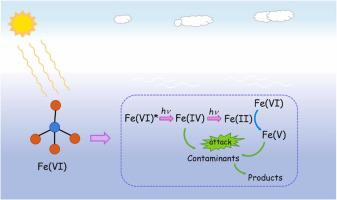
This paper presents a novel process of solar-ferrate(VI) [Fe(VI)] for micropollutant degradation. The solar-Fe(VI) process promoted micropollutant degradation compared with Fe(VI) alone and solar. The radical scavenging and probing experiment results suggested that Fe(V) and Fe(IV) but not reactive oxygen species were most likely involved in the solar-Fe(VI) process. Through building a kinetic model, Fe(IV) and Fe(V) were observed to play an equally significant role in the solar-Fe(VI) process. Afterward, the reaction mechanism of the photochemistry of Fe(VI) was elaborated. Fe(IV) formed from Fe(VI) photolysis and then decomposed to Fe(II) which reacted with Fe(VI) to form Fe(V). Furthermore, the effect of pH on carbamazepine (CBZ) degradation was studied and the quantum yields of Fe(VI) were determined, with (1.98±0.16)×10-3 mol∙einstein-1, (5.90±0.27)×10-4 mol∙einstein-1, and (1.66±0.14)×10-4 mol∙einstein-1 at pH 7.0, 8.0, and 9.0, respectively. Inorganic ions, including Cl-, HCO3-, and Br- displayed negligible influence on the CBZ degradation, whereas humic acid inhibited the CBZ degradation. Finally, the solar-Fe(VI) process exhibited good applicability in authentic waters and under different irradiation (natural sunlight, ultraviolet light, and visible light from solar cut-off emission). Overall, this study provides a new routine for efficient micropollutant elimination and reveals the photochemistry of Fe(VI).
中文翻译:

用于微污染物降解的新型太阳能驱动高铁酸盐 (VI) 活化系统:阐明 Fe(IV) 和 Fe(V) 的作用
本文介绍了一种用于微量污染物降解的太阳能高铁酸盐(VI) [Fe(VI)] 的新工艺。与单独的 Fe(VI) 和太阳能相比,太阳能-Fe(VI) 工艺促进了微污染物的降解。自由基清除和探测实验结果表明,Fe(V) 和 Fe(IV) 而不是活性氧最有可能参与太阳能-Fe(VI) 过程。通过建立动力学模型,观察到 Fe(IV) 和 Fe(V) 在太阳能-Fe(VI) 过程中发挥着同样重要的作用。随后阐述了Fe(VI)的光化学反应机理。Fe(IV) 由 Fe(VI) 光解形成,然后分解为 Fe(II),Fe(II) 与 Fe(VI) 反应形成 Fe(V)。此外,研究了pH对卡马西平(CBZ)降解的影响,并确定了Fe(VI)的量子产率,为(1.98±0.16)×10-3 mol∙einstein -1、(5.90±0.27)×10 -4 mol∙einstein -1和 (1.66±0.14)×10 -4 mol∙einstein -1分别在 pH 7.0、8.0 和 9.0 下。无机离子,包括Cl -、HCO 3 -和Br -对CBZ降解的影响可以忽略不计,而腐植酸抑制CBZ降解。最后,太阳能-Fe(VI)工艺在真实水域和不同辐照(自然阳光、紫外线和太阳截止发射的可见光)下表现出良好的适用性。总体而言,这项研究为有效去除微量污染物提供了一种新方法,并揭示了 Fe(VI) 的光化学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号