Chem Catalysis ( IF 11.5 ) Pub Date : 2022-06-22 , DOI: 10.1016/j.checat.2022.06.003 Xianhao Zhang , Huijuan Jing , Shiming Chen , Bing Liu , Liang Yu , Jianping Xiao , Dehui Deng
|
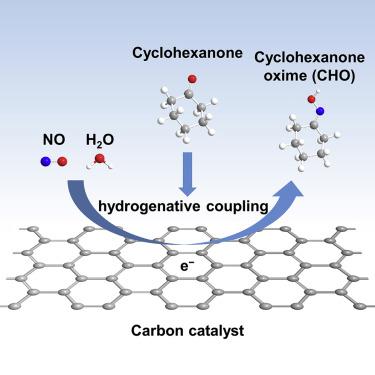
Cyclohexanone oxime (CHO), a key C=N organic compound in the manufacture of nylon 6, is conventionally synthesized via a multistep process that consumes expensive NH3 and H2 under harsh conditions. Herein, we report the direct electro-synthesis of CHO from low-cost NO, H2O, and cyclohexanone over a carbon catalyst under ambient conditions. A high Faradaic efficiency of 44.8% and a formation rate of 10.7 mg cm−2 h−1 for CHO were achieved at −0.4 V versus the reversible hydrogen electrode through optimizing the surface hydrophobicity of the catalyst to facilitate diffusion of reactants. Controlled experiments and 15N-labeling tests verified that the nitrogen in the CHO product originates from NO. Density functional theory calculations showed that the armchair edge of carbon could be the active site with a reaction mechanism following NO → NO∗ → HNO∗ → HNOH∗ → NH2OH → CHO. This work suggests a direct, green, and low-cost synthesis strategy for high-value CHO from pollutant NO.
中文翻译:

由 NO 直接电合成有价值的 C=N 化合物
环己酮肟 (CHO) 是尼龙 6 制造中的关键 C=N 有机化合物,通常通过多步工艺合成,该工艺在恶劣条件下消耗昂贵的 NH 3和 H 2 。在此,我们报告了在环境条件下在碳催化剂上由低成本的 NO、H 2 O 和环己酮直接电合成 CHO。通过优化催化剂的表面疏水性以促进反应物的扩散,与可逆氢电极相比,在-0.4 V 时,CHO 的法拉第效率为 44.8%,形成速率为 10.7 mg cm -2 h -1 。对照实验和15N标记测试证实CHO产物中的氮来源于NO。密度泛函理论计算表明,碳的扶手椅边缘可能是活性位点,其反应机理为NO→NO*→HNO*→HNOH*→NH 2 OH→CHO。这项工作提出了一种直接、绿色和低成本的从污染物 NO 合成高价值 CHO 的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号