Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2022-06-22 , DOI: 10.1016/j.cej.2022.137731 Wei Yang , Qiankun Han , Wenshi Li , Maosheng Wu , Jing Yao , Man Zhao , Xianmao Lu
|
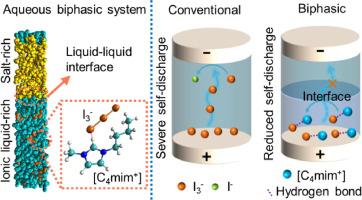
Redox-enhanced electrochemical capacitors (redox ECs) can boost the energy density of conventional ECs by introducing soluble redox species in the electrolyte to gain additional capacity via faradaic reactions. The shuttle effect of the added redox species such as polyiodides, however, leads to rapid self-discharge of redox ECs and severely limits their capability for long-term energy storage. Herein, we propose an ionic liquid (IL)-based aqueous biphasic system (ABS) as the electrolyte of iodide-based redox ECs to suppress the self-discharge. Based on molecular dynamics (MD) simulation and mechanistic analysis, we reveal that the liquid–liquid interface in the ABS blocks the diffusion of polyiodides and leads to reduced self-discharge rate. Specifically, EC cells with ABS electrolyte deliver much lower open circuit voltage (OCV) drop (0.7 V) and better energy retention (>32%) in 24 h than that of cells with conventional iodide-based electrolyte (OCV drop: 1.6 V, energy retention: 0%). This strategy is also successfully extended to the fabrication of ABS-based hydrogel electrolyte for solid-state redox ECs with low self-discharge rate. The results of this work not only provide a mechanistic approach to elucidating the effect of redox couples on the self-discharge of redox ECs, but also demonstrate an effective way of mitigating the shuttle effect via liquid–liquid interface in a biphasic electrolyte to reduce self-discharge.
中文翻译:

使用具有液-液界面的双相电解质抑制穿梭效应和氧化还原电化学电容器的自放电
氧化还原增强型电化学电容器 (redox ECs) 可以通过在电解液中引入可溶性氧化还原物质以通过法拉第反应获得额外容量,从而提高传统 ECs 的能量密度。然而,添加的氧化还原物质(如多碘化物)的穿梭效应导致氧化还原 EC 的快速自放电,并严重限制了它们的长期储能能力。在此,我们提出了一种基于离子液体(IL)的水性双相体系(ABS)作为碘化物基氧化还原EC的电解质来抑制自放电。基于分子动力学(MD)模拟和机理分析,我们发现ABS中的液-液界面阻碍了多碘化物的扩散并导致自放电率降低。具体来说,采用 ABS 电解质的 EC 电池可提供低得多的开路电压 (OCV) 下降 (0. 7 V)和比传统碘化物电解质电池更好的 24 小时能量保持率(>32%)(OCV 下降:1.6 V,能量保持率:0%)。该策略还成功地扩展到制备具有低自放电率的固态氧化还原 EC 的基于 ABS 的水凝胶电解质。这项工作的结果不仅提供了一种机制方法来阐明氧化还原电对对氧化还原 ECs 自放电的影响,而且还证明了一种通过双相电解质中的液-液界面减轻穿梭效应以减少自放电的有效方法。 -释放。该策略还成功地扩展到制备具有低自放电率的固态氧化还原 EC 的基于 ABS 的水凝胶电解质。这项工作的结果不仅提供了一种机制方法来阐明氧化还原电对对氧化还原 ECs 自放电的影响,而且还证明了一种通过双相电解质中的液-液界面减轻穿梭效应以减少自放电的有效方法。 -释放。该策略还成功地扩展到制备具有低自放电率的固态氧化还原 EC 的基于 ABS 的水凝胶电解质。这项工作的结果不仅提供了一种机制方法来阐明氧化还原电对对氧化还原 ECs 自放电的影响,而且还证明了一种通过双相电解质中的液-液界面减轻穿梭效应以减少自放电的有效方法。 -释放。











































 京公网安备 11010802027423号
京公网安备 11010802027423号