Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2022-06-22 , DOI: 10.1016/j.cej.2022.137744 Filipe Marques Mota , Omar Allam , Kyunghee Chae , Nur Aqlili Riana Che Mohamad , Seung Soon Jang , Dong Ha Kim
|
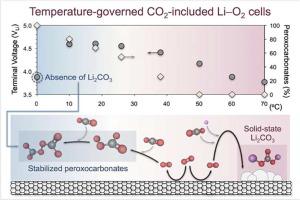
Practical lithium–oxygen batteries require a shift from pure O2 to air. CO2 traces, however, fundamentally alter the O2 electrochemistry towards Li2CO3 formation via peroxocarbonate intermediates in highly-solvating electrolytes e.g., tetraethylene glycol dimethyl ether (G4). Here, we reveal that operating temperatures (0∼70 °C) critically dictate Li2CO3-associated cell overcharges (4.60∼3.77 V), by determining temperature-dependent reaction kinetics and evolving species mobility, as analogously witnessed under pure O2-conditions. Against what is observed for Li2O2 formation in the Li–O2 cell, however, cell temperatures do not govern the crystallinity of the Li2CO3 discharge product. In agreement with experimental observations, comprehensive density functional theory calculations also uncover the effect of the temperature on the Li2CO3 precipitation mechanism and shed light on the dwindling stabilization of metastable peroxocarbonate intermediates during discharge at increasing cycling temperatures. On the other hand, during extended operation, the temperature-dependent reactants mobility in the viscous G4 electrolyte and remnant Li-deficient carbonate surfaces from precedent recharge steps play equally prominent roles on the Li2CO3 precipitation mechanism and the resulting cell capacity. Our study highlights the complexity of practical Li–O2 cells with temperature-dependent performances.
中文翻译:

实用性评估:含 CO2 的 Li-O2 电池的温度控制性能
实用的锂氧电池需要从纯 O 2转变为空气。然而,CO 2痕量从根本上改变了 O 2电化学,通过高溶剂化电解质(例如四甘醇二甲醚 (G4))中的过氧碳酸酯中间体形成Li 2 CO 3 。在这里,我们通过确定与温度相关的反应动力学和不断发展的物种迁移率,揭示了工作温度(0~70°C)对与 Li 2 CO 3相关的电池过充电(4.60~3.77 V)至关重要,正如在纯 O 2下所看到的那样-条件。与 Li 2 O 2的观察结果相反然而,在 Li-O 2电池中形成,电池温度并不控制 Li 2 CO 3放电产物的结晶度。与实验观察结果一致,综合密度泛函理论计算还揭示了温度对 Li 2 CO 3沉淀机制的影响,并阐明了在循环温度升高时放电过程中亚稳态过氧碳酸酯中间体的稳定性下降。另一方面,在延长运行期间,粘性 G4 电解质中与温度相关的反应物迁移率和来自先前充电步骤的残余缺锂碳酸盐表面对 Li 2 CO起着同样重要的作用3沉淀机理及由此产生的电池容量。我们的研究强调了具有温度依赖性性能的实用 Li-O 2电池的复杂性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号