当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile synthesis and phase stability of Cu-based Na2Cu(SO4)2·xH2O (x = 0–2) sulfate minerals as conversion type battery electrodes
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-06-22 , DOI: 10.1039/d2dt01830f Shashwat Singh 1, 2 , Audric Neveu 2 , K Jayanthi 3 , Tisita Das 4 , Sudip Chakraborty 4 , Alexandra Navrotsky 3 , Valérie Pralong 2 , Prabeer Barpanda 1, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-06-22 , DOI: 10.1039/d2dt01830f Shashwat Singh 1, 2 , Audric Neveu 2 , K Jayanthi 3 , Tisita Das 4 , Sudip Chakraborty 4 , Alexandra Navrotsky 3 , Valérie Pralong 2 , Prabeer Barpanda 1, 5
Affiliation
|
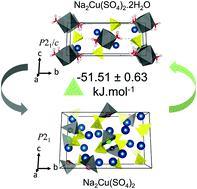
Mineral exploration forms a key approach for unveiling functional battery electrode materials. The synthetic preparation of naturally found minerals and their derivatives can aid in designing of new electrodes. Herein, saranchinaite Na2Cu(SO4)2 and its hydrated derivative kröhnkite Na2Cu(SO4)2·2H2O bisulfate minerals have been prepared using a facile spray drying route for the first time. The phase stability relation during the (de)hydration process was examined synergising in situ X-ray diffraction and thermochemical studies. Kröhnkite forms the thermodynamically stable phase as the hydration of saranchinaite to kröhnkite is highly exothermic (−51.51 ± 0.63 kJ mol−1). Structurally, kröhnkite offers a facile 2D pathway for Na+ ion migration resulting in 20 times higher total conductivity than saranchinaite at 60 °C. Both compounds exhibited a conversion redox mechanism for Li-ion storage with the first discharge capacity exceeding 650 mA h g−1 (at 2 mA g−1 vs. Li+/Li) upon discharge up to 0.05 V. Post-mortem analysis revealed that the presence of metallic Cu in the discharged state is responsible for high irreversibility during galvanostatic cycling. This study reaffirms the exploration of Cu-based polyanionic sulfates, which while having limited (de)insertion properties, can be harnessed for conversion-based electrode materials for batteries.
中文翻译:

Cu基Na2Cu(SO4)2·xH2O (x = 0-2)硫酸盐矿物作为转换型电池电极的简便合成和相稳定性
矿产勘探是揭示功能性电池电极材料的关键途径。天然矿物及其衍生物的合成制备有助于设计新电极。在此,首次采用简便的喷雾干燥法制备了沙兰奇石Na 2 Cu(SO 4 ) 2及其水合衍生物kröhnkite Na 2 Cu(SO 4 ) 2 ·2H 2 O 硫酸氢盐矿物。原位协同检查(脱水)过程中的相稳定性关系X射线衍射和热化学研究。Kröhnkite 形成热力学稳定相,因为 saranchinaite 水合为 kröhnkite 是高度放热的 (-51.51 ± 0.63 kJ mol -1 )。在结构上,kröhnkite 为 Na +离子迁移提供了一种简便的 2D 途径,导致在 60 °C 时总电导率比 saranchinaite 高 20 倍。两种化合物都表现出锂离子存储的转化氧化还原机制,首次放电容量超过 650 mA hg -1(在 2 mA g -1 vs. Li +/Li) 放电至 0.05 V。事后分析表明,放电状态下金属 Cu 的存在是恒电流循环过程中高度不可逆性的原因。这项研究重申了对铜基聚阴离子硫酸盐的探索,尽管铜基聚阴离子硫酸盐具有有限的(脱)插入特性,但可用于电池的基于转换的电极材料。
更新日期:2022-06-22
中文翻译:

Cu基Na2Cu(SO4)2·xH2O (x = 0-2)硫酸盐矿物作为转换型电池电极的简便合成和相稳定性
矿产勘探是揭示功能性电池电极材料的关键途径。天然矿物及其衍生物的合成制备有助于设计新电极。在此,首次采用简便的喷雾干燥法制备了沙兰奇石Na 2 Cu(SO 4 ) 2及其水合衍生物kröhnkite Na 2 Cu(SO 4 ) 2 ·2H 2 O 硫酸氢盐矿物。原位协同检查(脱水)过程中的相稳定性关系X射线衍射和热化学研究。Kröhnkite 形成热力学稳定相,因为 saranchinaite 水合为 kröhnkite 是高度放热的 (-51.51 ± 0.63 kJ mol -1 )。在结构上,kröhnkite 为 Na +离子迁移提供了一种简便的 2D 途径,导致在 60 °C 时总电导率比 saranchinaite 高 20 倍。两种化合物都表现出锂离子存储的转化氧化还原机制,首次放电容量超过 650 mA hg -1(在 2 mA g -1 vs. Li +/Li) 放电至 0.05 V。事后分析表明,放电状态下金属 Cu 的存在是恒电流循环过程中高度不可逆性的原因。这项研究重申了对铜基聚阴离子硫酸盐的探索,尽管铜基聚阴离子硫酸盐具有有限的(脱)插入特性,但可用于电池的基于转换的电极材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号